MMP-2-triggered, mitochondria-targeted PROTAC-PDT therapy of breast cancer and brain metastases inhibition
- PMID: 39613781
- PMCID: PMC11607387
- DOI: 10.1038/s41467-024-54854-2
MMP-2-triggered, mitochondria-targeted PROTAC-PDT therapy of breast cancer and brain metastases inhibition
Abstract
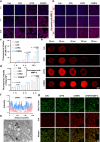
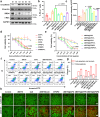
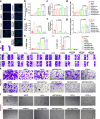
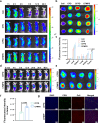
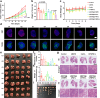
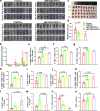
Proteolytic targeting chimera (PROTAC) technology is a protein-blocking technique and induces antitumor effects, with potential advantages. However, its effect is limited by insufficient distribution and accumulation in tumors. Herein, a transformable nanomedicine (dBET6@CFMPD) with mitochondrial targeting capacity is designed and constructed to combine PROTAC with photodynamic therapy (PDT). In this work, we demonstrate that dBET6@CFMPD exhibits great biodistribution and retention, and can induce potent antitumor response to suppress primary and metastatic tumors, becoming a nanomedicine with potential in cancer combination therapy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- Wang, F. et al. Dual-programmable semiconducting polymer NanoPROTACs for deep-tissue sonodynamic-ferroptosis activatable immunotherapy. Small20, 2306378 (2023). - PubMed
-
- Yang, C., Yang, Y., Li, Y., Ni, Q. & Li, J. Radiotherapy-triggered proteolysis targeting chimera prodrug activation in tumors. J. Am. Chem. Soc.145, 385–391 (2023). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

