Evidentiary basis of the first regulatory qualification of a digital primary efficacy endpoint
- PMID: 39613806
- PMCID: PMC11606965
- DOI: 10.1038/s41598-024-80177-9
Evidentiary basis of the first regulatory qualification of a digital primary efficacy endpoint
Abstract
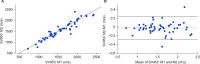
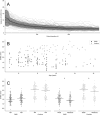
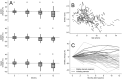
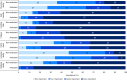
Stride velocity 95th centile (SV95C) is a wearable-derived endpoint representing the 5% fastest strides taken during everyday living. In July 2023, SV95C received European Medicines Agency (EMA) qualification for use as a primary endpoint in trials of patients with Duchenne muscular dystrophy (DMD) aged ≥ 4 years-becoming the first digital endpoint to receive such qualification. We present the data supporting this qualification, providing insights into the evidentiary basis of qualification as a digital clinical outcome assessment. Clinical trials, natural history studies, and patient surveys (ages 5 - 14 years) showed that SV95C is accurate, valid, reliable, sensitive, and clinically meaningful. SV95C significantly correlated with traditional DMD assessments, increased rapidly after steroid initiation (0.090 m/s 3 months post-treatment), and declined steadily in patients on stable steroid regimens. Compared with traditional assessments, SV95C demonstrated earlier sensitivity to disease progression (3 vs 9 months) and greater sensitivity at 12 months. Distribution- and anchor-based approaches revealed a change of - 0.10 to - 0.20 m/s as clinically meaningful. The EMA qualification of SV95C illustrates the willingness of regulators to accept novel digital endpoints for drug approval, setting an important precedent for the evidentiary basis of regulatory digital endpoint qualification that could transform clinical development in disorders affecting movement.
Keywords: Digital endpoints; Duchenne muscular dystrophy; Regulatory qualification; Stride Velocity 95th Centile; V3 framework; Wearables.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: L.S. is a member of scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, Biophytis, Cytokinetics, Dynacure, F. Hoffmann-La Roche Ltd, GeneTx Biotherapeutics, REGENXBIO, Santhera Pharmaceuticals, and Sarepta Therapeutics, Inc., has consulted for Pfizer and Affinia, conducts research funded by Novartis Gene Therapies (formerly AveXis), Biogen, and F. Hoffmann-La Roche Ltd, holds part of the patent WO2017129890A1 with no financial interest, and has provided consultancy services to SYSNAV. P.S. is an employee of and hold stocks in F. Hoffmann-La Roche Ltd. M.P. reports no disclosures relevant to the manuscript. A.M. reports no disclosures relevant to the manuscript. N.B. reports no disclosures relevant to the manuscript. V.A.S. provides intellectual consultancies and teaching activities for Biogen, F. Hoffmann-La Roche Ltd, Novartis, Lupin, Dyne Therapeutics, and PTC Therapeutics. C.V. reports participation in scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, PTC Therapeutics, F. Hoffmann-La Roche Ltd, Italfarmaco, and Sarepta Therapeutics, Inc., and is involved in research funded by Novartis Gene Therapies (formerly AveXis), Biogen, Sarepta Therapeutics, Inc., and F. Hoffmann-La Roche Ltd. U.S.S. is a member of scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, F. Hoffmann-La Roche Ltd, Pfizer, Santhera Pharmaceuticals, Sarepta Therapeutics, Inc., Italfarmaco, and PTC Therapeutics, and has received honoraria for invited talks or chair positions in scientific symposia from Novartis Gene Therapies (formerly AveXis), Biogen, F. Hoffmann-La Roche Ltd, Pfizer, Santhera Pharmaceuticals, Sarepta Therapeutics, Inc., Italfarmaco, and PTC Therapeutics. M.S. has provided consultancy services for and received honoraria (as a member of scientific advisory boards) from Biogen, F. Hoffmann-La Roche Ltd, and Novartis Gene Therapies (formerly AveXis). A.M.S. reports no disclosures relevant to the manuscript. S.C.P. reports participation in scientific advisory boards for EspeRare Foundation, Wave Life Sciences Ltd, Argenx, and Sarepta Therapeutics, Inc., and has provided consultancy service for Alia Therapeutics and LSC Lifesciences. M.T. has participated in scientific advisory boards for Biogen, PTC Therapeutics, F. Hoffmann-La Roche Ltd, and Sarepta Therapeutics, Inc., and has received honoraria for invited lectures from Biogen, Sarepta Therapeutics, Inc., and PTC Therapeutics. A.N. reports participation in scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, PTC Therapeutics, F. Hoffmann-La Roche Ltd, Italfarmaco, Pfizer, Dyne Therapeutics, and Sarepta Therapeutics, Inc., and is involved in research funded by Novartis Gene Therapies (formerly AveXis) and Biogen. P.F. reports no disclosures relevant to the manuscript. T. S. is an employee of Sarepta Therapeutics, Inc. and has stock and stock options. R.D.D. is Head of Clinical Development at Solid Biosciences, was previously employed at F. Hoffmann-La Roche Ltd, Santhera Pharmaceuticals, and Novartis, and has stock in Solid Biosciences and F. Hoffmann-La Roche Ltd. N.G. reports activities as a Data and Safety Monitoring Board member for Pfizer, Antisense Therapeutics, Wave Life Sciences Ltd, and Genethon. E.M. has served on clinical steering committees and/or as a consultant and received compensation from Italfarmaco, PTC Therapeutics, Sarepta Therapeutics, Inc., Santhera Pharmaceuticals, Pfizer Inc., F. Hoffmann-La Roche Ltd, Wave Life Sciences, NS Pharma, and Dyne Therapeutics, and is involved in research funded by Novartis Gene Therapies (formerly AveXis), Biogen, Sarepta Therapeutics, Inc., and F. Hoffmann-La Roche Ltd. V.S. has served on scientific advisory boards for Astellas Gene Therapies, Biogen, Edgewise Therapeutics, Ipsen, Kate Therapeutics, ML Bio Solutions, Novartis Gene Therapies, PepGen, F. Hoffmann-La Roche Ltd, Sanofi, Sarepta Therapeutics, Inc., Vertex Pharmaceuticals, and Wave Therapeutics, has received speaker honoraria from Pfizer, F. Hoffmann-La Roche Ltd, Sanofi, and Sarepta Therapeutics, Inc., has received grants for clinical research from Sarepta Therapeutics, Inc. and Sanofi, and has received support from the NIHR Newcastle Biomedical Research Centre. M.G.O. is an employee of and hold stocks in F. Hoffmann-La Roche Ltd. J.B. is an employee of and hold stocks in F. Hoffmann-La Roche Ltd. F.M. reports participation in scientific advisory boards for Novartis Gene Therapies (formerly AveXis), Biogen, F. Hoffmann-La Roche Ltd, Italfarmaco, Pfizer, Dyne Therapeutics, and Sarepta Therapeutics, Inc., and is involved in research funded by Novartis Gene Therapies (formerly AveXis), Biogen, Sarepta Therapeutics, Inc., and F. Hoffmann-La Roche Ltd. A.T. has nothing to disclose other than his employment at SYSNAV, a company that collaborated with the Institute of Myology to create ActiMyo®. M.A. was an employee of SYSNAV at the time that this manuscript was developed. D.E. has nothing to disclose other than his employment at SYSNAV, a company that collaborated with the Institute of Myology to create ActiMyo®.
Figures
References
-
- Markati, T. et al. Emerging therapies for Duchenne muscular dystrophy. Lancet Neurol.21, 814–829. 10.1016/S1474-4422(22)00125-9 (2022). - PubMed
-
- Kempf, L., Goldsmith, J. C. & Temple, R. Challenges of developing and conducting clinical trials in rare disorders. Am. J. Med. Genet. A.176, 773–783. 10.1002/ajmg.a.38413 (2018). - PubMed
-
- European Medicines Agency. Qualification opinion for stride velocity 95th centile as primary endpoint in studies in ambulatory Duchenne muscular dystrophy studies, https://www.ema.europa.eu/en/documents/scientific-guideline/qualificatio... (2023).
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

