Circulating tumor cells in breast cancer: clinical validity and utility
- PMID: 39613809
- PMCID: PMC11606964
- DOI: 10.1038/s41523-024-00706-7
Circulating tumor cells in breast cancer: clinical validity and utility
Abstract
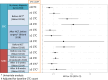
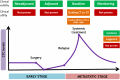
Circulating tumor cells (CTCs) have been extensively studied in breast cancer (BC), with large studies establishing CTCs as a robust prognostic biomarker in early and metastatic breast cancer (MBC). Several phase II and phase III trials have investigated the clinical utility of CTCs in BC. Here, we outline the current landscape for the use of CTCs in the clinic at different stages of BC, focusing first on early BC, then on MBC, with a particular focus on interventional clinical trials based on CTCs.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Cristofanilli, M. et al. Circulating Tumor Cells, Disease Progression, and Survival in Metastatic Breast Cancer. abbreviate. 8 www.nejm.org (2004). - PubMed
-
- Hortobagyi, G. N. et al. in AJCC Cancer Staging Manual 589–636 (Springer International Publishing, 2017).
Publication types
LinkOut - more resources
Full Text Sources

