MJF-14 proximity ligation assay detects early non-inclusion alpha-synuclein pathology with enhanced specificity and sensitivity
- PMID: 39613827
- PMCID: PMC11607433
- DOI: 10.1038/s41531-024-00841-9
MJF-14 proximity ligation assay detects early non-inclusion alpha-synuclein pathology with enhanced specificity and sensitivity
Abstract
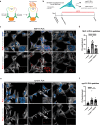
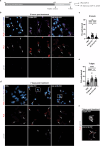
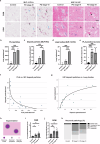
α-Synuclein proximity ligation assay (PLA) has proved a sensitive technique for detection of non-Lewy body α-synuclein aggregate pathology. Here, we describe the MJF-14 PLA, a new PLA towards aggregated α-synuclein with unprecedented specificity, using the aggregate-selective α-synuclein antibody MJFR-14-6-4-2 (hereafter MJF-14). Signal in the assay correlates with α-synuclein aggregation in cell culture and human neurons, induced by α-synuclein overexpression or pre-formed fibrils. Co-labelling of MJF-14 PLA and pS129-α-synuclein immunofluorescence in post-mortem cases of dementia with Lewy bodies shows that while the MJF-14 PLA reveals extensive non-inclusion pathology, it is not sensitive towards pS129-α-synuclein-positive Lewy bodies. In Parkinson's disease brain, direct comparison of PLA and immunohistochemistry with the MJF-14 antibody shows widespread α-synuclein pathology preceding the formation of conventional Lewy pathology. In conclusion, we introduce an improved α-synuclein aggregate PLA to uncover abundant non-inclusion pathology, which deserves future validation with brain bank resources and in different synucleinopathies.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Spillantini, M. G. & Goedert, M. The α-synucleinopathies: Parkinson’s disease, dementia with Lewy bodies, and multiple system atrophy. Ann. N. Y. Acad. Sci.920, 16–27 (2000). - PubMed
-
- de Lau, L. M. L. et al. Epidemiology of Parkinson’s disease. Lancet Neurol.5, 525–535 (2006). - PubMed
-
- Ascherio, A. & Schwarzschild, M. A. The epidemiology of Parkinson's disease: risk factors and prevention. Lancet Neurol.15, 1257–1272 (2016). - PubMed
-
- Polymeropoulos, M. H. Mutation in the α-Synuclein gene identified in families with Parkinson’s disease. Science276, 2045–2047 (1997). - PubMed
-
- Krüger, R. et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat. Genet.18, 106–108 (1998). - PubMed
Grants and funding
- SFB1286/A03/Deutsche Forschungsgemeinschaft (German Research Foundation)
- 12028.01/Michael J. Fox Foundation for Parkinson's Research (Michael J. Fox Foundation)
- 12028.01/Michael J. Fox Foundation for Parkinson's Research (Michael J. Fox Foundation)
- R28AA012725/U.S. Department of Health & Human Services | NIH | National Institute on Alcohol Abuse and Alcoholism (NIAAA)
- 1176607/Department of Health | National Health and Medical Research Council (NHMRC)
LinkOut - more resources
Full Text Sources

