Impact of haemoglobinA1c on platelet reactivity and cardiovascular outcomes in patients undergoing drug-eluting stent implantation
- PMID: 39613892
- PMCID: PMC11607451
- DOI: 10.1038/s41598-024-81537-1
Impact of haemoglobinA1c on platelet reactivity and cardiovascular outcomes in patients undergoing drug-eluting stent implantation
Abstract
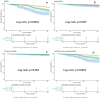
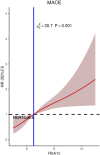
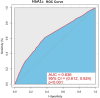
This study investigates the impact of hemoglobin A1c on platelet reactivity and cardiovascular outcomes in patients undergoing drug-eluting stent implantation. HbA1c levels were categorized into 3 groups: < 6.5%, 6.5-8.5%, and > 8.5%. ROC (resistance to clopidogrel) and ROA (resistance to aspirin) were calculated. The primary endpoint was a composite of MACE, including all-cause mortality, nonfatal MI, and ischemia-driven revascularization. The secondary endpoints comprised individual MACE components. The incidence of ROC was 9.3% (151 of 1621), whereas that of ROA was 16.5% (268 of 1621). The ROC for each of the 3 groups significantly increased with increasing HbA1c levels [4.3% vs. 7.1% vs. 10.1%, p = 0.006]; however, the ROA did not [16.4% vs. 17.7% vs. 14.3%, P = 0.694]. HbA1c > 8.5 was significantly associated with ROC (3.356 [1.231, 9.234], p = 0.009). Compared with the HbA1c < 6.5 subgroup, the HbA1c˃8.5 subgroup was significantly associated with MACE (3.142 [2.346, 4.206], < 0.001), nonfatal MI (2.297 [1.275, 4.137], P = 0.006) and ischemia-driven revascularization (3.845 [2.082, 7.101], p < 0.001), but not all-cause mortality (2.371 [0.551, 10.190], 0.246) at the 36-month follow-up. HbA1c levels were positively correlated with ROC, but the adverse cardiovascular events were driven by elevated HbA1c, constituting an argument to intensify glycemic control in subjects with diabetes after intracoronary stent placement.
Keywords: Atherosclerosis; Glycated hemoglobin; Percutaneous coronary intervention; Platelet reactivity.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: The study protocols were approval by the Ethics Committee of Jiading Branch of Shanghai General Hospital and performed in accordance with the Declaration of Helsinki. All participants provided written informed consent to participate in the study.
Figures

References
-
- Campo, G. et al. Long-term clinical outcome based on aspirin and clopidogrel responsiveness status after elective percutaneous coronary intervention: a 3T/2R (tailoring treatment with tirofiban in patients showing resistance to aspirin and/or resistance to clopidogrel) trial substudy. J. Am. Coll. Cardiol.56, 1447e1455 (2010). - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

