Mechanistic investigation of sustainable heme-inspired biocatalytic synthesis of cyclopropanes for challenging substrates
- PMID: 39613908
- PMCID: PMC11606945
- DOI: 10.1038/s42004-024-01371-4
Mechanistic investigation of sustainable heme-inspired biocatalytic synthesis of cyclopropanes for challenging substrates
Abstract
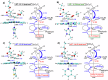
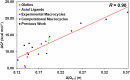
Engineered heme proteins exhibit excellent sustainable catalytic carbene transfer reactivities toward olefins for value-added cyclopropanes. However, unactivated and electron-deficient olefins remain challenging in such reactions. To help design efficient heme-inspired biocatalysts for these difficult situations, a systematic quantum chemical mechanistic study was performed to investigate effects of olefin substituents, non-native amino acid axial ligands, and natural and non-natural macrocycles with the widely used ethyl diazoacetate. Results show that electron-deficient substrate ethyl acrylate has a much higher barrier than the electron-rich styrene. For styrene, the predicted barrier trend is consistent with experimentally used heme analogue cofactors, which can significantly reduce barriers. For ethyl acrylate, while the best non-native axial ligand only marginally improves the reactivity versus the native histidine model, a couple of computationally studied macrocycles can dramatically reduce barriers to the level comparable to styrene. These results will facilitate the development of better biocatalysts in this area.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
Cyclopropanations via Heme Carbenes: Basic Mechanism and Effects of Carbene Substituent, Protein Axial Ligand, and Porphyrin Substitution.J Am Chem Soc. 2018 Feb 7;140(5):1649-1662. doi: 10.1021/jacs.7b09171. Epub 2018 Jan 24. J Am Chem Soc. 2018. PMID: 29268614 Free PMC article.
-
A comprehensive mechanistic investigation of sustainable carbene N-H insertion catalyzed by engineered His-ligated heme proteins.Catal Sci Technol. 2025 Jan 13;15(6):1802-1813. doi: 10.1039/d4cy00999a. eCollection 2025 Mar 17. Catal Sci Technol. 2025. PMID: 39882070 Free PMC article.
-
Biocatalytic Intramolecular C-H aminations via Engineered Heme Proteins: Full Reaction Pathways and Axial Ligand Effects.Chemistry. 2022 Oct 21;28(59):e202202006. doi: 10.1002/chem.202202006. Epub 2022 Aug 23. Chemistry. 2022. PMID: 35840505 Free PMC article.
-
Computational Mechanistic Investigations of Biocatalytic Nitrenoid C-H Functionalizations via Engineered Heme Proteins.Chembiochem. 2023 Sep 1;24(17):e202300260. doi: 10.1002/cbic.202300260. Epub 2023 Jul 27. Chembiochem. 2023. PMID: 37134298 Review.
-
Navigating the Unnatural Reaction Space: Directed Evolution of Heme Proteins for Selective Carbene and Nitrene Transfer.Acc Chem Res. 2021 Mar 2;54(5):1209-1225. doi: 10.1021/acs.accounts.0c00591. Epub 2021 Jan 25. Acc Chem Res. 2021. PMID: 33491448 Free PMC article. Review.
Cited by
-
Chemoenzymatic synthesis.Commun Chem. 2025 Mar 13;8(1):77. doi: 10.1038/s42004-025-01451-z. Commun Chem. 2025. PMID: 40082686 Free PMC article.
References
-
- Talele, T. T. The “Cyclopropyl Fragment” is a versatile player that frequently appears in preclinical/clinical drug molecules. J. Med. Chem.59, 8712–8756 (2016). - PubMed
-
- Wessjohann, L. A., Brandt, W. & Thiemann, T. Biosynthesis and metabolism of cyclopropane rings in natural compounds. Chem. Rev.103, 1625–1648 (2003). - PubMed
-
- Godula, K., Bärmann, H. & Donaldson, W. A. A simple and efficient synthesis of optically pure tricarbonyl(methyl 6-oxo-2,4-hexadienoate)iron. J. Org. Chem.66, 3590–3592 (2001). - PubMed
-
- Reichelt, A. & Martin, S. F. Synthesis and properties of cyclopropane-derived peptidomimetics. Acc. Chem. Res.39, 433–442 (2006). - PubMed
-
- Gnad, F. & Reiser, O. Synthesis and applications of β-aminocarboxylic acids containing a cyclopropane ring. Chem. Rev.103, 1603–1624 (2003). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

