Broadly conserved FlgV controls flagellar assembly and Borrelia burgdorferi dissemination in mice
- PMID: 39614093
- PMCID: PMC11607428
- DOI: 10.1038/s41467-024-54806-w
Broadly conserved FlgV controls flagellar assembly and Borrelia burgdorferi dissemination in mice
Abstract
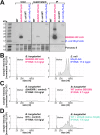
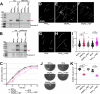
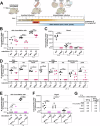
Flagella propel pathogens through their environments, yet are expensive to synthesize and are immunogenic. Thus, complex hierarchical regulatory networks control flagellar gene expression. Spirochetes are highly motile bacteria, but peculiarly, the archetypal flagellar regulator σ28 is absent in the Lyme spirochete Borrelia burgdorferi. Here, we show that gene bb0268 (flgV) in B. burgdorferi, previously and incorrectly annotated to encode the RNA-binding protein Hfq, is instead a structural flagellar component that modulates flagellar assembly. The flgV gene is broadly conserved in the flagellar superoperon alongside σ28 in many Spirochaetae, Firmicutes and other phyla, with distant homologs in Epsilonproteobacteria. We find that B. burgdorferi FlgV is localized within flagellar basal bodies, and strains lacking flgV produce fewer and shorter flagellar filaments and are defective in cell division and motility. During the enzootic cycle, flgV-deficient B. burgdorferi survive and replicate in Ixodes ticks but are attenuated for infection and dissemination in mice. Our work defines infection timepoints when spirochete motility is most crucial and implicates FlgV as a broadly distributed structural flagellar component that modulates flagellar assembly.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




Update of
-
Broadly conserved FlgV controls flagellar assembly and Borrelia burgdorferi dissemination in mice.bioRxiv [Preprint]. 2024 Jan 9:2024.01.09.574855. doi: 10.1101/2024.01.09.574855. bioRxiv. 2024. Update in: Nat Commun. 2024 Nov 29;15(1):10417. doi: 10.1038/s41467-024-54806-w. PMID: 38260563 Free PMC article. Updated. Preprint.
References
-
- Wolgemuth, C. W., Charon, N. W., Goldstein, S. F. & Goldstein, R. E. The flagellar cytoskeleton of the spirochetes. J. Mol. Microbiol Biotechnol.11, 221–227 (2006). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- FI2 GM133345/GM/NIGMS NIH HHS/United States
- R01 AI087946/AI/NIAID NIH HHS/United States
- ZIA LM594244/ImNIH/Intramural NIH HHS/United States
- R01 AI132818/AI/NIAID NIH HHS/United States
- R01 AI099094/AI/NIAID NIH HHS/United States
- S10 OD023603/OD/NIH HHS/United States
- 1Fi2GM133345/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- 1ZIAHD008995/U.S. Department of Health & Human Services | National Institutes of Health (NIH)
- ZIA HD001608/ImNIH/Intramural NIH HHS/United States
- ZIA HD008995/ImNIH/Intramural NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

