Tips from an expert panel on the development of a clinical research protocol
- PMID: 39614173
- PMCID: PMC11606108
- DOI: 10.1186/s12874-024-02315-1
Tips from an expert panel on the development of a clinical research protocol
Erratum in
-
Correction: Tips from an expert panel on the development of a clinical research protocol.BMC Med Res Methodol. 2024 Dec 13;24(1):300. doi: 10.1186/s12874-024-02438-5. BMC Med Res Methodol. 2024. PMID: 39668366 Free PMC article. No abstract available.
Abstract
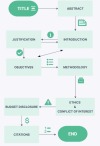
A research protocol is a document that outlines the proposed research idea and is submitted to funding agencies, institutions, or journals for approval. Writing a research protocol represents a challenge, particularly for early-career researchers. In this guide, we aim to provide detailed guidance with the key components and offer practical tips for crafting a research protocol in line with the various study designs. Specifically, the structure of a research protocol should contain the following items: (1) a title that is specific, catchy, and impressive within the word limitation; (2) an abstract that briefs the critical points of the study; (3) an introduction highlighting the study context from broad to narrow and defining the knowledge gap; (4) a justification underlining the significance of the proposed study; (5) Specific, Measurable, Attainable, Relevant, and Time-bound (SMART) objective(s) and aim(s); (6) a methodology covering seven sub-items, including [i] study design and settings, [ii] study subjects, [iii] sample size calculation and sampling, [iv] participants recruitment and follow-up, [v] questionnaire development, [vi] potential variables and outcomes, and [vii] data analysis plan; (7) dissemination of the results; (8) ethics and conflict of interests; (9) budgets analysis/ funding disclosure; and (10) references. This guide will give an overview of these steps and provide clear and concise tips on how to successfully draft a scientific protocol. With careful planning and appropriate guidance, it is possible to develop a well-structured and compelling protocol to obtain approval for the conduction of the study or funding from agencies, institutions, or organizations.
Keywords: Analysis planning; Clinical research; Grant proposal; Methods planning; Research protocol; Research question; Tips.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing of interests: The authors declare no competing interests.
Figures

References
-
- Creswell JW, Creswell JD. Research design. 5th ed. SAGE Publications; 2018.
-
- Denscombe M. Ground Rules for Social Research: Guidelines for Good Practice. 1st ed. UK: McGraw-Hill Education; 2009.
-
- Booth WC, Colomb GG, Williams JM, Bizup J, FitzGerald WT. The Craft of Research. 4th ed. The University of Chicago Press; 2016.
-
- Robinson KA, Brunnhuber K, Ciliska D, Juhl CB, Christensen R, Lund H, et al. Evidence-Based Research Series-Paper 1: What Evidence-Based Research is and why is it important? J Clin Epidemiol. 2021;129:151–7. 10.1016/j.jclinepi.2020.07.020. - PubMed
-
- Lund H, Juhl CB, Norgaard B, Draborg E, Henriksen M, Andreasen J, et al. Evidence-Based Research Series-Paper 2: Using an Evidence-Based Research approach before a new study is conducted to ensure value. J Clin Epidemiol. 2021;129:158–66. 10.1016/j.jclinepi.2020.07.019. - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous

