The first-in-class bispecific antibody IBI318 (LY3434172) targeting PD-1 and PD-L1 in patients with advanced tumors: a phase Ia/Ib study
- PMID: 39614368
- PMCID: PMC11606118
- DOI: 10.1186/s13045-024-01644-4
The first-in-class bispecific antibody IBI318 (LY3434172) targeting PD-1 and PD-L1 in patients with advanced tumors: a phase Ia/Ib study
Abstract
Background: There is an unmet clinical need to enhance the response rate and safety of anti-PD-1/PD-L1-based cancer immunotherapy (IO). Herein, we presented the clinical study of IBI318 (LY3434172), a first-in-class bispecific antibody (bsAb) targeting PD-1 and PD-L1, in patients with advanced tumors.
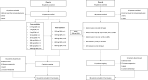
Methods: In this open-label, multicenter Phase Ia/Ib study of IBI318, the Phase Ia involved dose escalation and a safety dose expansion, while the Phase Ib focused on preliminary safety and efficacy evaluation in non-small cell lung cancer (NSCLC) and nasopharyngeal carcinoma (NPC). In Phase Ia, patients with advanced tumors received IBI318 doses ranging from 0.3 to 1200 mg every two weeks (Q2W) to determine the recommended Phase 2 dose (RP2D). In Phase Ib, NSCLC or NPC patients from five cohorts with varying treatment histories received IBI318 at the RP2D. The primary endpoint was safety and the secondary endpoints included efficacy assessed by investigators according to RECIST v1.1, pharmacokinetics, immunogenicity, and pharmacodynamics.
Results: From February 11, 2019, to January 25, 2022, a total of 103 eligible patients were enrolled (Phase Ia, n = 55; Phase Ib, n = 48). The median follow-up was 10.1 months (range 0.7-28.6). The RP2D was determined to be 300 mg Q2W. Treatment-related adverse events (TRAEs) of any grades occurred in 88 patients (85.4%), while 10 patients (9.7%) experienced grade ≥ 3 TRAEs. The objective response rate (ORR) was 15.5% and the disease control rate (DCR) was 49.5% in all patients. In Phase Ib, the confirmed ORR was 45.5% in treatment-naïve NSCLC patients and 30.0% in IO-naïve NPC patients who had failed or were intolerant to platinum-based treatments.
Conclusions: IBI318 demonstrated a favorable safety profile and preliminary efficacy in treatment-naïve NSCLC and IO-naïve NPC patients. Further clinical studies are needed to assess the full therapeutic potential of PD-1/PD-L1 dual inhibition with bsAbs.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The study protocol and all amendments were approved by the Institutional Review Boards of all participating institutions. All participants provided written informed consent. The study was performed in accordance with Good Clinical Practice guidelines and the Declaration of Helsinki. Consent for publication: Not applicable. Competing interests: Xiao-Xiao Lu, Yu-Ling Chen, Wen Zhang, Hui Zhou are employees of Innovent Biologics (Suzhou) Co. Ltd. Rui-Hua Xu reports speaker fees from Bristol Myers Squibb, Roche, MerckSerono, Hutchison, Hengrui, Junshi, Qilu, CPPC, Henlius, and participates on advisory board for Astellas, MSD, AstraZeneca, Junshi, Hengrui, BeiGene. Innovent, CPPC, and Keymed.
Figures


References
-
- Bagchi S, Yuan R, Engleman EG. Immune checkpoint inhibitors for the treatment of cancer: clinical impact and mechanisms of response and resistance. Annu Rev Pathol. 2021;16:223–49. - PubMed
-
- Sznol M, Ferrucci PF, Hogg D, Atkins MB, Wolter P, Guidoboni M, et al. Pooled analysis safety profile of nivolumab and ipilimumab combination therapy in patients with advanced melanoma. J Clin Oncol. 2017;35:3815–22. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

