Suppression of ADP-ribosylation reversal triggers cell vulnerability to alkylating agents
- PMID: 39615107
- PMCID: PMC11648251
- DOI: 10.1016/j.neo.2024.101092
Suppression of ADP-ribosylation reversal triggers cell vulnerability to alkylating agents
Abstract
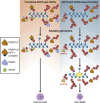
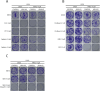
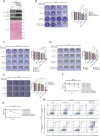
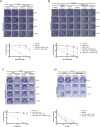
The ADP-ribosyl hydrolases PARG and ARH3 counteract PARP enzymatic activity by removing ADP-ribosylation. PARG and ARH3 activities have a synthetic lethal effect; however, the specific molecular mechanisms underlying this response remain unknown. Here, we show that the PARG and ARH3 synthetic lethality is enhanced further in the presence of DNA alkylating agents, suggesting that the inability to revert ADP-ribosylation primarily affects the repair of alkylated DNA bases. ARH3 knockout cells, treated with PARG inhibitor and alkylating genotoxins, accumulated single-stranded DNA and DNA damage, resulting in G2/M cell cycle arrest and apoptosis. Furthermore, we reveal a reduction in PARP1/PARP2 levels in ARH3-deficient cells treated with PARG inhibitor due to excessive ADP-ribosylation, which may contribute to alkylating agents' vulnerability. Collectively, these results uncover the potential of targeting ADP-ribosyl hydrolases in combination with alkylating agents for cancer therapy and provide insights into the mechanisms underlying the synthetic lethal effect.
Keywords: ADP-ribosyl hydrolases; ADP-ribosylation; ARH3; Alkylating drugs; Cancer; DNA damage; PARG inhibitor; PARP inhibitor.
Copyright © 2024. Published by Elsevier Inc.
Conflict of interest statement
Declaration of competing interest The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

