Activation of receptor-independent fluid-phase pinocytosis promotes foamy monocyte formation in atherosclerotic mice
- PMID: 39615283
- PMCID: PMC11647241
- DOI: 10.1016/j.redox.2024.103423
Activation of receptor-independent fluid-phase pinocytosis promotes foamy monocyte formation in atherosclerotic mice
Abstract
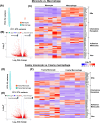
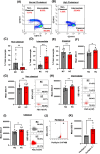
Atherosclerotic cardiovascular disease (ASCVD) is the leading cause of death worldwide. Clinical and experimental data demonstrated that circulating monocytes internalize plasma lipoproteins and become lipid-laden foamy cells in hypercholesterolemic subjects. This study was designed to identify the endocytic mechanisms responsible for foamy monocyte formation, perform functional and transcriptomic analysis of foamy and non-foamy monocytes relevant to ASCVD, and characterize specific monocyte subsets isolated from the circulation of normocholesterolemic controls and hypercholesterolemic patients. We hypothesized that activation of fluid-phase macropinocytosis contributes to foamy monocyte formation in vitro and in hypercholesterolemic mice in vivo. High resolution scanning electron microscopy (SEM) and quantification of FITC/TRITC-dextran internalization demonstrated macropinocytosis stimulation in human (THP-1) and wild type murine monocytes. Stimulation of macropinocytosis induced foamy monocyte formation in the presence of unmodified, native LDL (nLDL) and oxidized LDL (ox-LDL) in vitro. Genetic blockade of macropinocytosis (LysMCre+ Nhe1f/f) inhibited foamy monocyte formation in hypercholesterolemic mice in vivo and attenuated monocyte adhesion to atherosclerotic aortas ex vivo. Mechanistic studies identified NADPH oxidase 2 (Nox2)-derived superoxide anion (O2⋅-) as an important downstream signaling molecule stimulating macropinocytosis in monocytes. qRT-PCR identified CD36 as a major scavenger receptor that increases in response to lipid loading in monocytes and deletion of CD36 (Cd36-/-) inhibited foamy monocyte formation in hypercholesterolemic mice. Bulk RNA-sequencing characterized transcriptional differences between non-foamy and foamy monocytes versus macrophages. Finally, flow cytometry analysis of CD14 and CD16 expression demonstrated a significant increase in intermediate monocytes in hypercholesterolemic patients compared to normocholesterolemic controls. These results provide novel insights into the mechanisms of foamy monocyte formation and potentially identify new therapeutic targets for the treatment of atherosclerosis.
Keywords: Atherosclerosis; Hypercholesterolemia; Macropinocytosis; Monocyte; Ox-LDL; nLDL.
Copyright © 2024 The Authors. Published by Elsevier B.V. All rights reserved.
Conflict of interest statement
Declaration of competing interest The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be taken as a potential conflict of interest.
Figures




References
-
- Silverman M.G., Ference B.A., Im K., Wiviott S.D., Giugliano R.P., Grundy S.M., Braunwald E., Sabatine M.S. Association between lowering LDL-C and cardiovascular risk reduction among different therapeutic interventions: a systematic review and meta-analysis. JAMA. 2016;316:1289–1297. doi: 10.1001/jama.2016.13985. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

