Clinicopathological analysis of claudin 18.2 focusing on intratumoral heterogeneity and survival in patients with metastatic or unresectable gastric cancer
- PMID: 39615405
- PMCID: PMC11648117
- DOI: 10.1016/j.esmoop.2024.104000
Clinicopathological analysis of claudin 18.2 focusing on intratumoral heterogeneity and survival in patients with metastatic or unresectable gastric cancer
Abstract
Background: This study aimed to investigate the prevalence of claudin 18.2 (CLDN18.2) positivity, with a particular focus on intratumoral heterogeneity, and its association with clinicopathological features in metastatic or unresectable gastric cancer (GC).
Patients and methods: We investigated 400 patients who received systemic chemotherapy for unresectable, metastatic, or recurrent GC. Immunohistochemistry for CLDN18 (43-14A), human epidermal growth factor receptor 2 (HER2), programmed death-ligand 1 (PD-L1), and fibroblast growth factor receptor 2, as well as HER2 silver in situ hybridization (ISH), Epstein-Barr virus (EBV) ISH, and microsatellite instability testing were carried out. CD3+, CD8+, CD4+, and Foxp3-positive immune cell densities were calculated using digital image analysis.
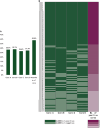
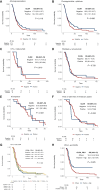
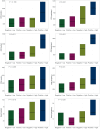
Results: In GC cases with any CLDN18.2 expression, more than half of the cases (61.3%) showed different expression results between four different tissue microarray (TMA) cores. When comparing CLDN18.2 status between whole tissue sections and the combined results from the four TMA cores, discrepancies were observed in only 2 out of 85 GC cases (2.4%), with 1 false positive and 1 false negative. After considering intratumoral heterogeneity, a CLDN18.2 positivity rate of 31.3% was observed among the 400 GC patients. CLDN18.2 positivity was rare in GCs located in the antrum (or lower third) and in HER2-positive cases but was common in EBV-positive GCs (P < 0.05). No differences in overall survival (OS) were observed according to CLDN18.2 positivity (P = 0.116). Additionally, there was no association between OS and CLDN18.2 positivity in patients treated with fluoropyrimidine plus platinum, chemotherapy plus trastuzumab, paclitaxel with or without ramucirumab, and immuno-oncologic agents. CLDN18.2-positive/PD-L1-high GCs showed statistically significantly longer OS than others (P = 0.025) and higher CD8+ T-cell densities in both the tumor center and periphery (P < 0.001).
Conclusions: Characterizing unresectable, metastatic, or recurrent GC with positive CLDN18.2 expression and evaluating intratumoral heterogeneity and prognostic implications of various therapeutics help advance treatment strategies and develop new therapies for patients with GC.
Keywords: claudin 18.2; gastric cancer; immune microenvironment; immunohistochemistry; intratumoral heterogeneity.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Figures

References
-
- Bang Y.J., Van Cutsem E., Feyereislova A., et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376(9742):687–697. - PubMed
-
- Rha S.Y., Oh D.Y., Yañez P., et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for HER2-negative advanced gastric cancer (KEYNOTE-859): a multicentre, randomised, double-blind, phase 3 trial. Lancet Oncol. 2023;24(11):1181–1195. - PubMed
-
- Janjigian Y.Y., Kawazoe A., Bai Y., et al. Pembrolizumab plus trastuzumab and chemotherapy for HER2-positive gastric or gastro-oesophageal junction adenocarcinoma: interim analyses from the phase 3 KEYNOTE-811 randomised placebo-controlled trial. Lancet. 2023;402(10418):2197–2208. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

