Misfolding of transthyretin in vivo is controlled by the redox environment and macromolecular crowding
- PMID: 39615680
- PMCID: PMC11732491
- DOI: 10.1016/j.jbc.2024.108031
Misfolding of transthyretin in vivo is controlled by the redox environment and macromolecular crowding
Abstract
Transthyretin (TTR) amyloidosis is a progressive disorder characterized by peripheral neuropathy, autonomic dysfunction, and cardiomyopathy. The precise mechanism by which TTR misfolds and forms fibrils in vivo remains incompletely understood, posing challenges to the development of effective therapeutics. In this study, we reveal that the recently identified nonnative pathological species of TTR (NNTTR), which is enriched in the plasma of ttr-val30met gene carriers, exhibits strong amyloidogenic properties, making it a promising therapeutic target. Notably, we demonstrate that NNTTR formation is dependent on an intermolecular disulfide bond and can be promoted by oxidative conditions while being effectively suppressed by reducing agents. The formation of this disulfide bond is incompatible with the native TTR fold, thereby necessitating structural flexibility. We further show that this required flexibility can be constrained using tetramer-stabilizing drugs, thereby suppressing NNTTR formation. Interestingly, the flexibility is also hindered by macromolecular crowding, and NNTTR formation is strongly suppressed by the high protein concentration in plasma. This suppression is released upon dilution, which thus promotes NNTTR formation in areas with lower protein content, highlighting a potential link to the interstitial space, brain, and vitreous body of the eye, where TTR-amyloid is frequently observed. In summary, we demonstrate that NNTTR displays strong amyloidogenic features, underscoring its potential as a therapeutic target. We identify the redox environment and macromolecular crowding as key modulatory factors. Our findings propose a mechanistic explanation for TTR misfolding and suggest a novel therapeutic approach.
Keywords: amyloid; cysteine; disulfide; macromolecular crowding; redox; transthyretin.
Copyright © 2024 The Authors. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
Conflict of interest S. W. J., I. A., and A. O. are the owners of a patent application based on the described findings. PCT/SE2024/050271. The other authors declare that they have no conflicts of interest with the contents of this article.
Figures








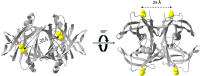

References
Publication types
MeSH terms
Substances
Supplementary concepts
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

