Metabolic control of luteinizing hormone-responsive ovarian steroidogenesis
- PMID: 39615688
- PMCID: PMC11732475
- DOI: 10.1016/j.jbc.2024.108042
Metabolic control of luteinizing hormone-responsive ovarian steroidogenesis
Abstract
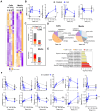
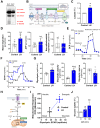
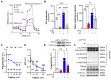
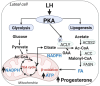
The pituitary gonadotropin luteinizing hormone (LH) is the primary stimulus for ovulation, luteal formation, and progesterone synthesis, regardless of species. Despite increased awareness of intracellular signaling events initiating the massive production of progesterone during the reproductive cycle and pregnancy, critical gaps exist in our knowledge of the metabolic and lipidomic pathways required for initiating and maintaining luteal progesterone synthesis. Using untargeted metabolomics and metabolic flux analysis in primary steroidogenic luteal cells, evidence is provided for rapid LHCGR-stimulation of metabolic pathways leading to increased glycolysis and oxygen consumption. Treatment with LH stimulated posttranslational modifications of enzymes involved in de novo lipogenesis. Mechanistic studies implicated a crucial role for de novo fatty acid synthesis and fatty acid oxidation in energy homeostasis, LHCGR/PKA signaling, and, consequently, progesterone production. These findings reveal novel hormone-sensitive metabolic pathways essential for maintaining LHCGR/PKA signaling and steroidogenesis. Understanding hormonal control of metabolic pathways in steroidogenic cells may help elucidate approaches for improving ovarian function and successful reproduction or identifying metabolic targets for developing nonhormonal contraceptives.
Keywords: G protein-coupled receptor (GPCR); cyclic AMP; lipogenesis; metabolism; ovary; progesterone; protein kinase A (PKA).
Published by Elsevier Inc.
Conflict of interest statement
Conflict of interest The authors declare that they have no conflicts of interest with the contents of this article.
Figures



References
-
- Talbott H., Davis J.S. In: The Life Cycle of the Corpus Luteum. Meidan R., editor. Springer International Publishing; Cham: 2017. Lipid droplets and metabolic pathways regulate steroidogenesis in the corpus luteum; pp. 57–78.
-
- Niswender G.D., Juengel J.L., McGuire W.J., Belfiore C.J., Wiltbank M.C. Luteal function: the estrous cycle and early pregnancy. Biol. Reprod. 1994;50:239–247. - PubMed
-
- Niswender G.D., Juengel J.L., Silva P.J., Rollyson M.K., McIntush E.W. Mechanisms controlling the function and life span of the corpus luteum. Physiol. Rev. 2000;80:1–29. - PubMed
-
- Borman S.M., Chaffin C.L., Schwinof K.M., Stouffer R.L., Zelinski-Wooten M.B. Progesterone promotes oocyte maturation, but not ovulation, in nonhuman primate follicles without a gonadotropin Surge1. Biol. Reprod. 2004;71:366–373. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

