Adverse effects of the aesthetic use of botulinum toxin and dermal fillers on the face: a narrative review
- PMID: 39616095
- PMCID: PMC11745296
- DOI: 10.1016/j.abd.2024.04.007
Adverse effects of the aesthetic use of botulinum toxin and dermal fillers on the face: a narrative review
Abstract
Objective: To evaluate the adverse effects of facial aesthetic treatments using botulinum toxin and biomaterial implants.
Methods: The bibliographic research for this narrative review considered articles published in journals from the Medline, Pubmed, Embase and Lilacs databases with the following terms: "dermal fillers AND complications, vascular complications AND dermal fillers, adverse reaction, AND toxin botulinum and adverse reaction AND dermal fillers". Inclusion criteria were articles available in English on adverse events with the aesthetic use of botulinum toxin and dermal fillers/biostimulators.
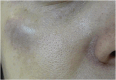
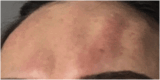
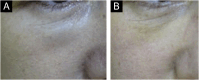
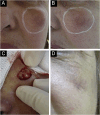
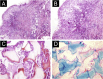
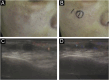
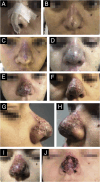
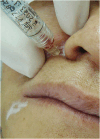
Results: The demonstration of complications increases simultaneously with the progressive performance of facial aesthetic procedures. Quantitative statistics of the procedures and the countries that use them are skillfully classified, as well as the prosperity trends of these procedures. Complications do not receive the same relevance. There is a deficiency in dissemination of the information by the scientific community, or in other words, there is a publication bias in favor of successful results as opposed to adverse events.
Conclusion: The lack of knowledge about complications arising from so widely publicized and performed procedures prevents the development of evidence-based guidelines. Complications in aesthetic procedures have become a public health problem, an epidemic that occurs under the supervision of health authorities. Mandatory reporting of adverse events occurring in aesthetic procedures that require medical care aims to fill this gap. With reliable and technical data, it will be possible to identify the causes and perform interventions capable of minimizing irreversible sequelae and deaths. Complications should be promptly recognized by the dermatologist so that, when possible, they can be reversed or adequately managed.
Keywords: Biocompatible materials; Botulinum toxins, type A; Dermal fillers; Disease notification; Drug-related side effects and adverse reactions; Health planning guidelines; Hyaluronic acid.
Copyright © 2024. Published by Elsevier España, S.L.U.
Figures




References
-
- American Society of Aesthetic Plastic Surgery [Internet] [cited 2023 Jan 03] Available from: https://www.isaps.org/discover/about-isaps/global-statistics/reports-and....
-
- Código de Ética Médica - Cap. I- IX e X Cap. III – art. 20 Cap. VI – art. 46 Cap. VIII – art. 58, 63, 68 e 72 Cap. XIII – art. 116.
-
- Di Santis E.P. Universidade Federal de São Paulo; São Paulo: 2018. Mortes relacionadas à lipoaspiração no Brasil entre 1987 e 2015 [tese]
-
- Kroumpouzos G., Kassir M., Gupta M., Patil A., Goldus M. Complications of botulinum toxin A: an update review. J Cosmet Dermatol. 2021;20:1585–1590. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

