Photochemically-enabled, post-translational production of C-terminal amides
- PMID: 39616180
- PMCID: PMC11608224
- DOI: 10.1038/s41467-024-51005-5
Photochemically-enabled, post-translational production of C-terminal amides
Abstract
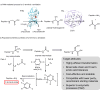
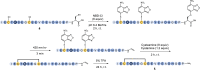
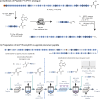
C-terminal α-amidated peptides are attractive therapeutic targets, but preparative methods to access amidated pharmaceuticals are limited both on lab and manufacturing-scale. Here we report a straightforward and scalable approach to the C-terminal α-amidation of peptides and proteins from cysteine-extended polypeptide precursors. This amidation protocol consists of three highly efficient steps: 1) selective cysteine thiol substitution with a photolabel, 2) photoinduced decarboxylative elimination and 3) enamide cleavage by simple acidolysis or inverse electron demand Diels-Alder reaction. We provide a blueprint for applying this protocol to the semi-recombinant production of therapeutically relevant targets where gram scale C-terminal α-amidation is achieved in a photoflow reactor on a recombinantly prepared peptide YY analogue and a GLP-1/amylin co-agonist precursor peptide. Robust performance of this reaction cascade in flow highlights the potential of this chemistry to enable amidated drug leads to enter development that would not be viable on commercial scale using existing technology.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: All authors except S. C. W. are employees and minor shareholders of Novo Nordisk A/S. D.H., F.W., W.F.J.H., S.C.W., B.M.W., N.C., A.R.M., M.R.H are co-inventors of a patent application (WO23105074A1) describing photochemically enabled amidations.
Figures


References
-
- Henninot, A., Collins, J. C. & Nuss, J. M. The current state of peptide drug discovery: back to the future? J. Med. Chem.61, 1382–1414 (2018). - PubMed
-
- Drucker, D. J. Advances in oral peptide therapeutics. Nat. Rev. Drug Discov.19, 277–289 (2020). - PubMed
-
- Bray, B. L. Large-scale manufacture of peptide therapeutics by chemical synthesis. Nat. Rev. Drug Discov.2, 587–593 (2003). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

