A computational model that integrates unrestricted callus growth, mechanobiology, and angiogenesis can predict bone healing in rodents
- PMID: 39616231
- PMCID: PMC11608358
- DOI: 10.1038/s41598-024-80502-2
A computational model that integrates unrestricted callus growth, mechanobiology, and angiogenesis can predict bone healing in rodents
Abstract
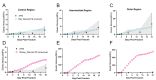
We present a computational model that integrates mechanobiological regulations, angiogenesis simulations and models natural callus development to simulate bone fracture healing in rodents. The model inputs include atomic force microscopy values and micro-computed tomography on the first-day post osteotomy, which, combined with detailed finite element modeling, enables scrutinizing mechanical and biological interactions in early bone healing and throughout the healing process. The model detailed mesenchymal stem cell migration patterns, which are essential for tissue transformation and vascularization during healing, indicating the vital role of blood supply in the healing process. The model predicted bone healing in rodents (n = 48) over 21 days, matching daily tissue development with histological evidence. The developed computational model successfully predicts tissue formation rates and stiffness, reflecting physiological callus growth, and offers a method to simulate the healing process, potentially extending to humans in the future.
Keywords: Angiogenesis; Bone healing mechanobiology; Computational modeling; Plate fixation; Rat fracture model; Unrestricted callus growth.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures








References
-
- Isaksson, H. Recent advances in mechanobiological modeling of bone regeneration. Mech. Res. Commun.42, 22–31 (2012). - DOI
MeSH terms
LinkOut - more resources
Full Text Sources

