Precursor-free synthesis of carbon quantum dots and carbon microparticles in supercritical acetone
- PMID: 39616236
- PMCID: PMC11608273
- DOI: 10.1038/s42004-024-01367-0
Precursor-free synthesis of carbon quantum dots and carbon microparticles in supercritical acetone
Abstract
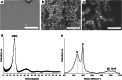
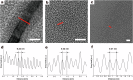
Carbon quantum dots (CQDs) have recently received a lot of attention due to their unique physical properties, and their environmentally friendly features such as low toxicity and high biocompatibility. Supercritical fluids, which possess unusual properties such as high solubility, high diffusivity, low viscosity and zero surface tension, are now commonly used particularly in the fields of electronic, chemical and materials science and engineering. Here, we synthesise carbon nano/microparticles in supercritical acetone, in which neither external molecules nor starting materials are dissolved/dispersed. We find that carbon microparticles and nano structures such as graphene quantum dots (GQDs), carbon nano onions (CNOs) and elongated carbon nano onions (eCNOs) are self-assembled via thermal decomposition of acetone under its supercritical conditions. We also find that the carbon microparticles are in fact formed by GQDs, CNOs and eCNOs, the microparticles being physically resolved into GQDs, CNOs and eCNOs with sonication. The fluorescence features of the carbon nano structures are clarified, noting that no photobleaching was observed for at least one month. The present result may well lead to the development of facile bottom-up methodologies for synthesising nano materials in solvents under their supercritical conditions without using any external precursors/starting materials.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures


References
-
- Kroto, H. W., Heath, J. R., O’Brien, S. C., Curl, R. F. & Smalley, R. E. C60: buckminsterfullerene. Nature318, 162–163 (1985).
-
- Povie, G., Segawa, Y., Nishihara, T., Miyauchi, Y. & Itami, K. Synthesis of a carbon nanobelt. Science356, 172–175 (2017). - PubMed
-
- Omachi, H., Nakayama, T., Takahashi, E., Segawa, Y. & Itami, K. Initiation of carbon nanotube growth by well-defined carbon nanorings. Nat. Chem.5, 572–576 (2013). - PubMed
-
- Iijima, S. Synthesis of carbon nanotubes. Nature354, 56–58 (1991).
-
- Geim, A. K. & Novoselov, K. S. The rise of graphene. Nat. Mater.6, 183–191 (2007). - PubMed
LinkOut - more resources
Full Text Sources

