CD22 blockade exacerbates neuroinflammation in Neuromyelitis optica spectrum disorder
- PMID: 39616380
- PMCID: PMC11607829
- DOI: 10.1186/s12974-024-03305-2
CD22 blockade exacerbates neuroinflammation in Neuromyelitis optica spectrum disorder
Abstract
Background: Neuromyelitis optica spectrum disorder (NMOSD) is an autoantibody-triggered central nervous system (CNS) demyelinating disease that primarily affects the spinal cord, optic nerves and brainstem. Among the first responders to CNS injury, microglia are prominent players that drive NMOSD lesion formation. However, the key molecular switches controlling the detrimental activity of microglia in NMOSD are poorly understood. CD22 governs the activity of innate and adaptive immunity. In this study, we investigated to what extent and by what mechanisms CD22 may modulate microglial activity, neuroinflammation and CNS lesion formation.
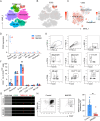
Methods: To determine the expression profile of CD22 in NMOSD, we performed single-cell sequencing and flow cytometry analysis of immune cells from human peripheral blood. We investigated the potential effects and mechanisms of CD22 blockade on microglial activity, leukocyte infiltration and CNS demyelination in a mouse model of NMOSD induced by injection of NMOSD patient serum-derived AQP4-IgG and human complement.
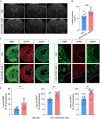
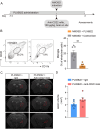
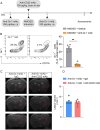
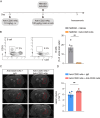
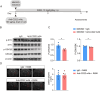
Results: Single-cell sequencing and flow cytometry analysis revealed that CD22 was expressed in B cells, neutrophils, monocytes and microglia-derived exosomes in human peripheral blood from NMOSD patients and controls (n = 5 per group). In a mouse model of NMOSD, CD22 was expressed in B cells, neutrophils, monocytes and microglia (n = 8 per group). In NMOSD mice, CD22 blockade significantly increased the number of CNS lesions, astrocyte loss and demyelination, accompanied by increased inflammatory activity and phagocytosis in microglia. Furthermore, the detrimental effects of CD22 blockade were significantly alleviated in NMOSD mice subjected to depletion of microglia or Gr-1+ myeloid cells, suggesting the involvement of microglia and peripheral Gr-1+ myeloid cells. Additionally, CD22 blockade also led to significantly reduced phosphorylation of SYK and GSK3β in NMOSD. Notably, the detrimental effects of CD22 blockade were greatly diminished in NMOSD mice receiving the phosphorylated SYK inhibitor R406.
Conclusions: Our findings revealed a previously unrecognized role of CD22 as a key molecular switch that governs the detrimental effects of microglia and Gr-1+ myeloid cells in NMOSD, which paves the way for the future design of immune therapies for NMOSD.
Keywords: CD22; Demyelination; Microglia; Neuroinflammation; Neuromyelitis optica spectrum disorder.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal procedures were approved by the Animal Experiments Ethical Committee of Tianjin Medical University and carried out in accordance with the Revised Guide for the Care and Use of Laboratory Animals. Written informed consent for blood donation was obtained from all control subjects and NMOSD patients, in line with the local ethical committee guidelines, and the studies were conducted under Research Ethics Committee approval (IRB2022-YX-084-01). Consent for publication: No applicable. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
Grants and funding
- 82301522/National Natural Science Foundation of China
- 82301495/National Natural Science Foundation of China
- 82201498/National Natural Science Foundation of China
- 82372651/National Natural Science Foundation of China
- 82225015/National Natural Science Foundation of China
- 82271378/National Natural Science Foundation of China
- 22JCQNJC00500/Tianjin Municipal Science and Technology Bureau
- 2021KJ215/Tianjin Municipal Education Commission
- 2021ZD035/Tianjin Municipal Education Commission
- BX20240097/National Postdoctoral Program for Innovative Talents
- 2024M750652/China Postdoctoral Science Foundation
- TJWJ2023QN011/Tianjin Municipal Health Commission Science and Technology Project
- 2021ZD0202400/National Key Research and Development Program of China
LinkOut - more resources
Full Text Sources
Miscellaneous

