Sitagliptin eye drops prevent the impairment of retinal neurovascular unit in the new Trpv2+/- rat model
- PMID: 39616390
- PMCID: PMC11607821
- DOI: 10.1186/s12974-024-03283-5
Sitagliptin eye drops prevent the impairment of retinal neurovascular unit in the new Trpv2+/- rat model
Abstract
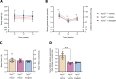
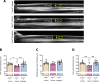
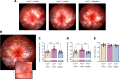
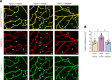
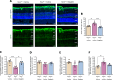
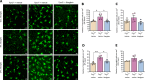
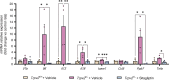
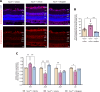
Impaired function of the retinal neurovascular unit (NVU) is an early event in diabetic retinopathy (DR). It has been previously shown that topical delivery of the dipeptidyl peptidase-4 (DPP-4) inhibitor sitagliptin can protect against diabetes-mediated dysfunction of the retinal NVU in the db/db mouse. The aim of the present study was to examine whether sitagliptin could prevent the DR-like lesions within the NVU of the new non-diabetic model of DR, the Trpv2 knockout rat (Trpv2+/-). For that purpose, at 3 months of age, Trpv2+/- rats were topically treated twice daily for two weeks with sitagliptin or PBS-vehicle eyedrops. Trpv2+/+ rats treated with vehicle served as the control group. Body weight and glycemia were monitored. Optical coherence tomography recordings, fundus images and retinal samples were obtained to evaluate sitagliptin effects. The results revealed that sitagliptin eye drops had no effect on body weight or glycemia. Vehicle-treated Trpv2+/- rats exhibited retinal thinning and larger diameters of major retinal blood vessels, upregulation of inflammatory factors and oxidative markers, glial activation and formation of acellular capillaries. However, topical administration of sitagliptin significantly prevented all these abnormalities. In conclusion, sitagliptin eye drops exert a protective effect against DR-like lesions in Trpv2+/- rats. Our results suggest that sitagliptin eye drops carry significant potential to treat not only early-stages of DR but also other diseases with impairment of the NVU unrelated to diabetes.
Keywords: DPP-4 inhibitor; Diabetes; Diabetic retinopathy; Sitagliptin; Trpv2.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: Animal experiments were approved by the Animal Welfare Ethical Review Body (AWERB) of Queen’s University Belfast. The protocol complied with the UK Home Office Animals (Scientific Procedures) Act 1986 (project license, PPL2888) and the Association for Research in Vision and Ophthalmology (ARVO) statement for the use of animals in ophthalmology and vision research. Competing interests: Two of the authors (Cristina Hernández and Rafael Simó) are inventors of the patent PCT/EP2017/060234, which is related to use of dipeptidyl peptidase-4 inhibitors (sitagliptin) for topical eye treatment of retinal neurodegenerative diseases.
Figures

References
-
- Wong TY, Cheung CM, Larsen M, Sharma S, Simó R. Diabetic retinopathy. Nat Rev Dis Primers. 2016;2:16012. 10.1038/nrdp.2016.12. - PubMed
-
- Hernández C, Bogdanov P, Corraliza L, et al. Topical administration of GLP-1 receptor agonists prevents retinal neurodegeneration in experimental diabetes. Diabetes. 2016;65(1):172–87. 10.2337/db15-0443. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

