HepB-CpG vs HepB-Alum Vaccine in People With HIV and Prior Vaccine Nonresponse: The BEe-HIVe Randomized Clinical Trial
- PMID: 39616603
- PMCID: PMC11610526
- DOI: 10.1001/jama.2024.24490
HepB-CpG vs HepB-Alum Vaccine in People With HIV and Prior Vaccine Nonresponse: The BEe-HIVe Randomized Clinical Trial
Abstract
Importance: Nonresponse to hepatitis B vaccine is common among people with HIV, resulting in vulnerability to infection with hepatitis B virus (HBV).
Objective: To compare the seroprotection response achieved with a 2-dose (noninferiority, 10% margin) and a 3-dose hepatitis B vaccine with a cytosine phosphoguanine adjuvant (HepB-CpG vaccine) vs a conventional 3-dose hepatitis B vaccine with an aluminum hydroxide adjuvant (HepB-alum vaccine) in people with HIV and prior nonresponse to HepB-alum vaccine.
Design, setting, and participants: This phase 3, open-label, randomized clinical trial included people with HIV receiving antiretroviral therapy (CD4 cell count ≥100 cells/μL and HIV RNA <1000 copies/mL) without past or present serological evidence of having HBV or a response to hepatitis B vaccine. From December 2020 to February 2023, 561 adults were enrolled in the study at 41 sites in 10 countries in Africa, Asia, North America, and South America with follow-up for the primary outcome analysis through September 4, 2023.
Interventions: Participants were randomly assigned to receive 2 doses of HepB-CpG vaccine administered intramuscularly at weeks 0 and 4; 3 doses of HepB-CpG vaccine administered intramuscularly at weeks 0, 4, and 24; or 3 doses of HepB-alum vaccine administered intramuscularly at weeks 0, 4, and 24.
Main outcomes and measures: The primary outcome was a seroprotection response to hepatitis B vaccine (defined as level of antibody titer against hepatitis B surface antigen [HBsAg] ≥10 mIU/mL) at week 12 for the 2-dose regimen (8 weeks after dose 2) and at week 28 for 3-dose regimens (4 weeks after dose 3). Key secondary outcomes included seroprotection response at additional time points, antibody titer against HBsAg, and adverse events within 4 weeks of hepatitis B vaccination.
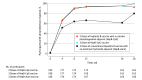
Results: Of 561 participants included in the analysis (median age, 46 years [IQR, 31-56 years]); 64% were male; 17% of participants were Asian, 42% were Black, and 35% were White), a seroprotection response was achieved in 93.1% who received 2 doses of HepB-CpG vaccine (n = 174), in 99.4% who received 3 doses of HepB-CpG vaccine (n = 169), and in 80.6% who received 3 doses of HepB-alum vaccine (n = 165). The stratified difference in seroprotection response between the 2-dose HepB-CpG vaccine group and the 3-dose HepB-alum vaccine group was 12.5% (97.5% CI, 4.1%-20.9%), achieving noninferiority and indicating superiority. The 3-dose HepB-CpG vaccine regimen was superior to the 3-dose HepB-alum vaccine regimen (stratified difference in seroprotection response, 18.4% [repeated 97.5% CI, 10.4%-26.2%]). By week 12, more than 90% of participants who received HepB-CpG vaccine achieved a seroprotection response. The 3-dose regimen of HepB-CpG vaccine achieved a higher proportion of participants with antibody titer against HBsAg greater than 1000 mIU/mL (78.1%) vs the other 2 regimen groups (26.4% for 2 doses of HepB-CpG vaccine and 35.2% for 3 doses of HepB-alum vaccine). No unexpected safety issues were observed.
Conclusions and relevance: Among people with HIV and nonresponse to prior hepatitis B vaccination, both the 2-dose and 3-dose regimens of HepB-CpG vaccine achieved a superior seroprotection response compared with 3 doses of HepB-alum vaccine.
Trial registration: ClinicalTrials.gov Identifier: NCT04193189.
Conflict of interest statement
Figures




Comment on
-
Overcoming Hepatitis B Vaccine Nonresponsiveness.JAMA. 2025 Jan 28;333(4):291-292. doi: 10.1001/jama.2024.24028. JAMA. 2025. PMID: 39616602 No abstract available.
References
-
- World Health Organization . Global hepatitis report 2024: action for access in low- and middle-income countries. Published April 9, 2024. Accessed November 6, 2024. https://www.who.int/publications/i/item/9789240091672
Publication types
MeSH terms
Substances
Associated data
Grants and funding
- UM1 AI069463/AI/NIAID NIH HHS/United States
- UM1 AI069432/AI/NIAID NIH HHS/United States
- UM1 AI069419/AI/NIAID NIH HHS/United States
- UM1 AI069518/AI/NIAID NIH HHS/United States
- UM1 AI154466/AI/NIAID NIH HHS/United States
- UM1 AI069465/AI/NIAID NIH HHS/United States
- UM1 AI069453/AI/NIAID NIH HHS/United States
- UL1 TR002243/TR/NCATS NIH HHS/United States
- UM1 AI069501/AI/NIAID NIH HHS/United States
- UM1 AI106701/AI/NIAID NIH HHS/United States
- UL1 TR000124/TR/NCATS NIH HHS/United States
- UM1 AI069418/AI/NIAID NIH HHS/United States
- UM1 AI069476/AI/NIAID NIH HHS/United States
- UM1 AI069471/AI/NIAID NIH HHS/United States
- UM1 AI069424/AI/NIAID NIH HHS/United States
- U01 AI069439/AI/NIAID NIH HHS/United States
- U01 AI069432/AI/NIAID NIH HHS/United States
- UL1 TR001881/TR/NCATS NIH HHS/United States
- UM1 AI069534/AI/NIAID NIH HHS/United States
- U01 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI069399/AI/NIAID NIH HHS/United States
- UM1 AI069470/AI/NIAID NIH HHS/United States
- UM1 AI068634/AI/NIAID NIH HHS/United States
- P30 AI036214/AI/NIAID NIH HHS/United States
- UM1 AI069481/AI/NIAID NIH HHS/United States
- UM1 AI069511/AI/NIAID NIH HHS/United States
- UM1 AI069423/AI/NIAID NIH HHS/United States
- UM1 AI069494/AI/NIAID NIH HHS/United States
- UM1 AI069496/AI/NIAID NIH HHS/United States
- UM1 AI069412/AI/NIAID NIH HHS/United States
- UM1 AI069439/AI/NIAID NIH HHS/United States
- UL1 TR002384/TR/NCATS NIH HHS/United States
- UM1 AI069452/AI/NIAID NIH HHS/United States
- UM1 AI108568/AI/NIAID NIH HHS/United States
- UM1 AI068636/AI/NIAID NIH HHS/United States
- P30 AI050410/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

