FUNCTIONAL IMMUNOPHENOTYPING FOR PRECISION THERAPIES IN SEPSIS
- PMID: 39617419
- PMCID: PMC12447363
- DOI: 10.1097/SHK.0000000000002511
FUNCTIONAL IMMUNOPHENOTYPING FOR PRECISION THERAPIES IN SEPSIS
Abstract
Sepsis remains a significant cause of morbidity and mortality worldwide. Although many more patients are surviving the acute event, a substantial number enters a state of persistent inflammation and immunosuppression, rendering them more vulnerable to infections. Modulating the host immune response has been a focus of sepsis research for the past 50 years, yet novel therapies have been few and far between. Although many septic patients have similar clinical phenotypes, pathways affected by the septic event differ not only between individuals but also within an individual over the course of illness. These differences ultimately impact overall immune function and response to treatment. Defining the immune state, or endotype, of an individual is critical to understanding which patients will respond to a particular therapy. In this review, we highlight current approaches to define the immune endotype and propose that these technologies may be used to "prescreen" individuals to determine which therapies are most likely to be beneficial.
Copyright © 2024 by the Shock Society.
Conflict of interest statement
S.B., K.E.R., C.C.C., L.L.M., and R.S.H. have a patent pending for an application of whole blood ELISpot. The specifics of the assay for which the patent is being sought are not discussed in this article. All other authors have no relevant conflicts of interest to disclose.
Figures

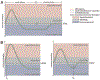
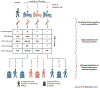
References
-
- Fleischmann C, Scherag A, Adhikari NKJ, et al. Trialists IF of AC: assessment of global incidence and mortality of hospital-treated Sepsis. Current estimates and limitations. Am J Respir Crit Care Med. 2016;193(3):259–272. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

