Neuropathological and cerebrospinal fluid correlates of choroid plexus inflammation in progressive multiple sclerosis
- PMID: 39617422
- PMCID: PMC12145902
- DOI: 10.1111/bpa.13322
Neuropathological and cerebrospinal fluid correlates of choroid plexus inflammation in progressive multiple sclerosis
Abstract
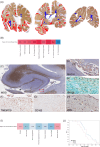
Among the intrathecal inflammatory niches where compartmentalized inflammation persists and plays a pivotal role in progressive multiple sclerosis (MS), choroid plexus (CP) has recently received renewed attention. To better characterize the neuropathological/molecular correlates of CP in progressive MS and its potential link with other brain inflammatory compartments, such as perivascular spaces and leptomeninges, the levels, composition and phenotype of CP immune infiltration in lateral ventricles of the hippocampus were examined in 40 post-mortem pathologically confirmed MS and 10 healthy donors, using immunochemistry/immunofluorescence and in-situ sequencing. Significant inflammation was detected in the CP of 21 out of the 40 MS cases (52%). The degree of CP inflammation was found correlated with: number of CP macrophages (R: 0.878, p = 1.012 x 10-13) and high frequency of innate immune cells expressing the markers MHC-class II, CD163, CD209, CD11c, TREM2 and TSPO; perivascular inflammation (R: 0.509, p = 7.921 x 10-4), and less with meningeal inflammation (R: 0.365, p = 0.021); number of active lesions (R: 0.51, p: 3.524 x 10-5). However, it did not significantly correlate with any clinical/demographic characteristics of the examined population. In-situ sequencing analysis of gene expression in the CP of 3 representative MS cases and 3 controls revealed regulation of inflammatory pathways mainly related to 'type 2 immune response', 'defense to infections', 'antigen processing/presentation'. Analysis of 78 inflammatory molecules in paired post-mortem CSF, the levels of fibrinogen (R: 0.640, p = 8.752 x 10-6), PDGF-bb (R: 0.470, p = 0.002), CXCL13 (R: 0.428, p = 0.006) and IL15 (R: 0.327, p = 0.040) were correlated with extent of CP inflammation. Elevated fibrinogen and complement deposition were found in CP and in underlying subependymal periventricular areas, according to "surface-in" gradient associated with concomitant prominent microglia activation. CP inflammation, predominantly characterized by innate immunity, represents another key determinant of intrathecal, compartmentalised inflammation persisting in progressive MS, which may be possibly activated by fibrinogen and influence periventricular pathology, even without substantial association with clinical features.
Keywords: choroid plexus; gradient; inflammation; multiple sclerosis.
© 2024 The Author(s). Brain Pathology published by John Wiley & Sons Ltd on behalf of International Society of Neuropathology.
Conflict of interest statement
The authors declare no conflicts of interest related to this study.
Figures





References
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

