A stearate-rich diet and oleate restriction directly inhibit tumor growth via the unfolded protein response
- PMID: 39617788
- PMCID: PMC11671534
- DOI: 10.1038/s12276-024-01356-2
A stearate-rich diet and oleate restriction directly inhibit tumor growth via the unfolded protein response
Abstract
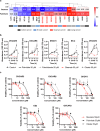
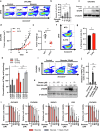
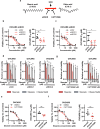
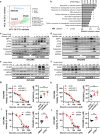
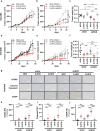
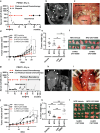
Fatty acids are known to have significant effects on the properties of cancer cells. Therefore, these compounds have been incorporated into therapeutic strategies. However, few studies have examined the effects of individual fatty acids and their interactions in depth. This study analyzed the effects of various fatty acids on cancer cells and revealed that stearic acid, an abundant saturated fatty acid, had a stronger inhibitory effect on cell growth than did palmitic acid, which is also an abundant saturated fatty acid, by inducing DNA damage and apoptosis through the unfolded protein response (UPR) pathway. Intriguingly, the negative effects of stearate were reduced by the presence of oleate, a different type of abundant fatty acid. We combined a stearate-rich diet with the inhibition of stearoyl-CoA desaturase-1 to explore the impact of diet on tumor growth. This intervention significantly reduced tumor growth in both ovarian cancer models and patient-derived xenografts (PDXs), including those with chemotherapy resistance, notably by increasing stearate levels while reducing oleate levels within the tumors. Conversely, the negative effects of a stearate-rich diet were mitigated by an oleate-rich diet. This study revealed that dietary stearate can directly inhibit tumor growth through mechanisms involving DNA damage and apoptosis mediated by the UPR pathway. These results suggest that dietary interventions, which increase stearic acid levels while decreasing oleic acid levels, may be promising therapeutic strategies for cancer treatment. These results could lead to the development of new cancer treatment strategies.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: E.N. has received research funding from Sumitomo Pharma Co., Ltd. The other authors declare that they have no competing interests.
Figures

References
-
- Yang, J. et al. High-fat diet promotes colorectal tumorigenesis through modulating gut microbiota and metabolites. Gastroenterology162, 135–149.e2 (2022). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials

