AAV delivery strategy with mechanical support for safe and efficacious cardiac gene transfer in swine
- PMID: 39617804
- PMCID: PMC11609281
- DOI: 10.1038/s41467-024-54635-x
AAV delivery strategy with mechanical support for safe and efficacious cardiac gene transfer in swine
Abstract
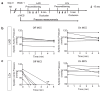
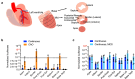
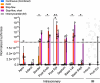
Adeno-associated virus-based gene therapy is a promising avenue in heart failure treatment, but has shown limited cardiac virus uptake in humans, requiring new approaches for clinical translation. Using a Yorkshire swine ischemic heart failure model, we demonstrate significant improvement in gene uptake with temporary coronary occlusions assisted by mechanical circulatory support. We first show that mechanical support during coronary artery occlusions prevents hemodynamic deterioration (n = 5 female). Subsequent experiments show that coronary artery occlusions during gene delivery improve gene transduction, while adding coronary sinus occlusion (Stop-flow) further improves gene expression up to >1 million-fold relative to conventional intracoronary infusion. Complete survival during and after delivery (n = 10 female, n = 10 male) further indicates safety of the approach. Improved cardiac gene expression correlates with virus uptake without an increase in extra-cardiac expression. Stop-flow delivery of virus-sized gold nanoparticles exhibits enhanced endothelial adherence and uptake, suggesting a mechanism independent of virus biology. Together, utilizing mechanical support for cardiac gene delivery offers a clinically-applicable strategy for heart failure-targeted therapies.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: Part of this study was supported by a research grant from Abiomed, Inc. to the institution. K.I. serves as the principal investigator on the grant from Abiomed, Inc. K.I. received an honorarium from Abiomed, Inc. and served as a consultant for Pfizer, Inc., and Gordian Biotechnology. T.S. and T.K. were supported by A-CURE Research Fellowship supported by Abiomed, Inc.. K.I. and R.J.H. have a patent, “SYSTEMS AND METHODS FOR LEFT VENTRICULAR UNLOADING IN BIOLOGIC THERAPY OR VECTORED GENE THERAPY” Publication number: 20200305888. All other authors declare no competing interests.
Figures





References
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

