Efficacy and safety of guselkumab in European patients with palmoplantar pustulosis: A multi-center, single-arm clinical trial (GAP study)
- PMID: 39618912
- PMCID: PMC11607598
- DOI: 10.1016/j.jdin.2024.09.001
Efficacy and safety of guselkumab in European patients with palmoplantar pustulosis: A multi-center, single-arm clinical trial (GAP study)
Abstract
Background: Palmoplantar pustulosis (PPP) is a chronic inflammatory skin disorder that affects palms and soles. Patients suffer significant pain, itching, and daily activity impairment. Guselkumab, an interleukin-23 inhibitor, has been approved for PPP treatment in Japan. However, there is no effective therapy licensed for PPP in Europe and the USA.
Objective: To explore the efficacy and safety of guselkumab in patients with moderate-to-severe PPP in the Caucasian population.
Methods: A multicenter, single-arm, phase II study involving 50 patients with moderate-to-severe PPP treated with 100 mg guselkumab subcutaneously for 24 weeks was conducted (GAP). Primary endpoint was the reduction of palmoplantar-pustulosis psoriasis area and severity index (PPPASI) at week 24 compared to baseline. Secondary endpoints included physician-assessed and patient-reported measures. Serum samples were taken for exploratory studies.
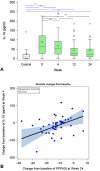
Results: The primary endpoint was met with a significant median PPPASI reduction by 59.6% at week 24 compared to baseline (P < .001). The proportions of patients achieving PPPASI-50 and PPPASI-75 at week 24 were 66.0% and 34.0%, respectively. Median dermatology life quality index dropped from 15 at baseline to 5 at week 24 (P < .001). Week 4 changes in interleukin-19 serum levels predicted week 24 clinical response.
Conclusion: Guselkumab may be a promising therapeutic option for PPP in Caucasian patients.
Keywords: IL-23; PPPASI; guselkumab; interleukin-19; multicenter trial; palmoplantar pustular psoriasis; palmoplantar pustulosis; smoking.
© 2024 by the American Academy of Dermatology, Inc. Published by Elsevier Inc.
Conflict of interest statement
Dr Wilsmann-Theis has been an advisor, speaker, or investigator for Abbvie, Almirall, Amgen, Biogen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, GlaxoSmithKline, Hexal, Incyte, Janssen-Cilag, Leo Pharma, Eli Lilly, Medac, Merck Sharp & Dohme Corp., Novartis, Pfizer, and UCB Pharma. Author Patt has been investigator for and/ or received grants from AbbVie, AnaptysBio, Boehringer Ingelheim, Bristol-Myers Squibb, Celgene, Galderma, Incyte, Janssen, LEO Pharma, Novartis Pharma, OM Pharma, Pfizer, Regeneron, and UCB Pharma. Dr Pinter has served as an advisor and/or paid speaker for and/or participated in clinical trials sponsored by: AbbVie, Almirall-Hermal, Amgen, Biogen Idec, Biontec, BMS, Boehringer-Ingelheim, Celgene, Celltrion, GSK, Eli-Lilly, Eva Pharma, Galderma, Hexal, Incyte, Janssen-Cilag, Klinge Pharma LEO-Pharma, MC2, Medac, Merck Serono, Mitsubishi, Moonlake, MSD, Novartis, Pascoe, Pfizer, Tigercat Pharma, Regeneron, Roche, Sandoz Biopharmaceuticals, Sanofi-Genzyme, Schering-Plough, UCB Pharma, and Zuellig Pharma. Dr Gerdes has been an advisor and/or received speakers' honoraria and/or received grants and/or participated in clinical trials of the following companies: AbbVie, Acylering, Affibody AB, Akari Therapeutics Plc, Almirall-Hermal, Amgen, Anaptys Bio, Argenx BV, AstraZeneca AB, Bioskin, Bristol-Myers Squibb, Boehringer-Ingelheim, Celgene, Dermira, Eli Lilly, Galderma, Hexal AG, Incyte Inc., Janssen-Cilag, Johnson & Johnson, Klinge Pharma, Kymab, Leo Pharma, Medac, Neubourg Skin Care GmbH, Novartis, Pfizer, Principia Biopharma, Regeneron Pharmaceutical, Sandoz Biopharmaceuticals, Sanofi-Aventis, and UCB Pharma. N Magnolo has received honoraria as an advisor, speaker, and/or consultant AbbVie, Almirall, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Celgene, Janssen-Cilag, La Roche-Posay, LEO Pharma, Lilly, Novartis, Pfizer, Dr Wolff, and UCB Pharma. Drs Németh, Paul, Hüffmeier has no conflict of interest to declare. Author Schmitz has no conflict of interest to declare. Dr Paul has no conflict of interest to declare. Dr Augustin has served as a consultant, lecturer or researcher, and/or has received research grants from companies manufacturing drugs for psoriasis, including AbbVie, Almirall, Amgen, Biogen, Boehringer Ingelheim, BMS, Celgene, Centocor, Eli Lilly, Galderma, Hexal, Janssen, Klinge, LEO, Medac, MSD, Mylan B.V., Novartis, Pfizer, Sandoz, Takeda, UCB, and Viatris. Dr Staubach has received research grants, travel grants, consulting or lecturer's honoraria from Abbvie, Allergika, Almirall-Hermal, Amgen, Avene, Unna Akademie, Biocryst, BMS, Boehringer-Ingelheim, Celgene, CSL-Behring, Eli-Lilly, Galderma, GSK, Janssen, Klinge, LEO-Pharma, L'Oreal, Novartis, Octapharma, Pfizer, Pharming, Regeneron, Shire, Takeda, Sanofi-Genzyme, and UCB Pharma. Dr Weyergraf has served as a speaker, advisor and/or researcher for AbbVie, Almirall, Amgen, Arctic Bioscience, Biogen, Bristol-Myers-Squibb, Celgene, Hermal, Janssen, LEO, Lilly, Novartis, Pfizer, Sanofi, and UCB. Dr Wolk has received research grants or contracts for clinical trials (payment to her institution), support for attending congresses, scientific awards, consulting fees or honoraria for participation in advisory boards, or honoraria for lectures for one or more of the following: Celgene/Amgen, Celgene/Bristol Myers Squibb, Charité Research Organization, Flexopharm, Janssen-Cilag, Novartis Pharma, Sanofi–Aventis, TFS Trial Form Support, University hospital Magdeburg, European HS foundation (EHSF), and the Symposium on Hidradenitis Suppurativa Advances (SHSA); she also has an non-financial relationship to the HS task force of the German Consortium for Dermatological Research (ADF). Dr Sabat has received research grants, clinical trial contracts, scientific awards, or honoraria for consulting, participation in advisory boards, or for lectures for one or more of the following: AbbVie, Almirall Hermal, Amgen, Bayer Schering Pharma, Boehringer Ingelheim Pharma, Bruno Bloch Stiftung, Celgene/Amgen, Celgene/Bristol Myers Squibb, Charité Research Organisation, CSL Behring, ICON, IQVIA RDS, Incyte, Janssen-Cilag/Janssen Research & Development, MoonLake Immunotherapeutics, Novartis Pharma, Parexel, Rheinischen Friedrich-Wilhelms-Universität Bonn, Sanofi–Aventis, TFS, UCB Biopharma, Universitätsmedizin Greifswald, and Wundnetz Berlin-Brandenburg e. V. Dr Mӧßner has been an advisor and/or received speakers’ honoraria and/or received grants and/or participated in clinical trials of the following companies: Abbvie, Allmirall, Biogen IDEC GmbH, Böhringer-Ingelheim, Celgene, Janssen-Cilag GmbH, Leo Pharma GmbH, Eli Lilly and Company, Merck Serono GmbH, MSD SHARP & DOHME GmbH, Novartis Pharma GmbH, Pfizer GmbH, and UCB.
Figures


References
-
- Wilsmann-Theis D., Jacobi A., Frambach Y., et al. Palmoplantar pustulosis - a cross-sectional analysis in Germany. Dermatol Online J. 2017;23(4):1–11. - PubMed
-
- Bissonnette R., Suarez-Farinas M., Li X., et al. Based on molecular profiling of gene expression, palmoplantar pustulosis and palmoplantar pustular psoriasis are highly related diseases that appear to Be distinct from psoriasis vulgaris. PLoS One. 2016;11(5) doi: 10.1371/journal.pone.0155215. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

