Vortioxetine in the Treatment of Major Depressive Disorder Among Working Patients in Routine Clinical Practice: An Analysis of a Post-Marketing Surveillance Study in South Korea
- PMID: 39619493
- PMCID: PMC11608001
- DOI: 10.2147/NDT.S478804
Vortioxetine in the Treatment of Major Depressive Disorder Among Working Patients in Routine Clinical Practice: An Analysis of a Post-Marketing Surveillance Study in South Korea
Abstract
Background: Patients with major depressive disorder (MDD) experience depressive symptoms such as anhedonia as well as cognitive dysfunction which can subsequently impair their work performance.
Purpose: To assess the effectiveness and safety of vortioxetine in working patients with MDD in South Korea.
Patients and methods: This was a subgroup analysis of a prospective, multicenter, non-interventional, non-comparative post-marketing surveillance (PMS) study. Vortioxetine-naïve patients aged >18 years who were administered with vortioxetine were followed for up to 24±2 weeks. Working patients were defined as those who were working or studying full- (≥6 hours/day) or part-time (<6 hours/day) at baseline. Effectiveness and adverse events (AEs), assessed by both clinician and patient-reported measured, were analyzed.
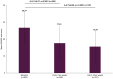
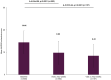
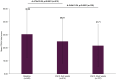
Results: A total of 1082 working patients (mean age: 39.56 years) were included in the subgroup analysis. Clinically significant improvements in depressive symptoms, including anhedonia, were observed over the 24 weeks of follow-up, with mean scores for the total Montgomery-Asberg Depression Rating Scale (MADRS) and anhedonia subscale both significantly decreasing from baseline by mean±standard deviation (SD) of 9.73±9.08 and 5.37±5.24 points, respectively, at 24 weeks (both p<0.001 vs baseline). The vast majority of patients (80.01%) treated with vortioxetine also showed improvements in mental health symptoms over the 24 weeks, measured using the Clinical Global Impression - Improvement (CGI-I) scores. Significant improvements in cognitive symptoms were also observed over the study period, measured by the Korean Version of the Perceived Deficits Questionnaire-Depression as well as Digit Symbol Substitution Test (all p<0.0001 from baseline at Visits 2 and 3). Vortioxetine was well tolerated in working patients, with the respective rates of any AEs and serious AEs being 18.67% and 1.20%.
Conclusion: Working patients treated with vortioxetine had improvements in their depressive symptoms (including anhedonia), cognitive function and performance. Vortioxetine was found to be well tolerated in this study.
Keywords: South Korea; major depressive disorder; non-interventional; post-marketing surveillance; vortioxetine; work performance.
© 2024 Moon et al.
Conflict of interest statement
This was a non-interventional study with the protocol and all data, including all adverse events, having been shared with and reviewed by MFDS. The study was funded by H. Lundbeck A/S, whose personnel contributed to the data analysis, review of the data, and review of the manuscript: Elin Heldbo Reines and Daniel Oudin Astrom are employees of H. Lundbeck A/S, Valby, Denmark. Minah Lee and Gayoung Kim are employees of Lundbeck Korea Co., Ltd., Seoul, Korea. Michael Adair was a former employee of H. Lundbeck A/S, Valby, Denmark during the course of the study. The authors report no other conflicts of interest in this work.
Figures
References
-
- World Health Organisation. Depressive disorder (Depression); 2023. Available from: https://www.who.int/news-room/fact-sheets/detail/depression. Accessed March 8, 2024.
-
- GBD 2019 Mental Disorders Collaborators. Global, regional, and national burden of 12 mental disorders in 204 countries and territories, 1990-2019: a systematic analysis for the global burden of disease study 2019. Lancet Psychiatry. 2022;9(2):137–150. doi:10.1016/s2215-0366(21)00395-3 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources