Influence of Co Doping on Copper Nanoclusters for CO2 Electroreduction
- PMID: 39619520
- PMCID: PMC11603234
- DOI: 10.1021/acsomega.4c07514
Influence of Co Doping on Copper Nanoclusters for CO2 Electroreduction
Abstract
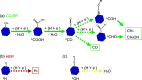
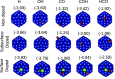
Copper stands out as one of the few metals capable of reducing carbon dioxide (CO2) beyond carbon monoxide (CO) and formic acid (HCOOH). Furthermore, substitutional doping in nanoclusters (NCs) has been expected to enhance their catalytic performance, even though our atomistic understanding of the influence of dopants is far from complete. Here, we investigate the effects induced by cobalt (Co) substitution doping in the Cu55 NC on the electroreduction of CO2 using density functional theory calculations combined with the computational hydrogen electrode model. We found that the replacement of a single copper atom in Cu55 by Co is energetically favorable, and it induces a drastic change in the density of states, for example, the appearance of a sharp peak near the Fermi level. The presence of a dopant atom on the surface increases the adsorption strength for all reaction intermediates, while also changing the preference of the adsorption site for selected species. The presence of the dopant atom on the surface of the particle hinders the production of CO in favor of more reduced products such as methane and methanol. From our analysis, it was observed that the catalyst will not suffer from poisoning by the OH species. However, our calculations predict that the catalysts will also enhance the formation of hydrogen in a competing reaction.
© 2024 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


References
-
- Jain A. K.; O’Sullivan M.; Jones M. W.; et al. Global carbon budget 2022. Earth Syst. Sci. Data 2022, 14, 4811–4900. 10.5194/essd-14-4811-2022. - DOI
-
- Yaashikaa P.; Kumar P. S.; Varjani S. J.; Saravanan A. A review on photochemical, biochemical and electrochemical transformation of CO2 into value-added products. J. CO2 Util. 2019, 33, 131–147. 10.1016/j.jcou.2019.05.017. - DOI
-
- Komarala E. P.; Alkhoori A. A.; Zhang X.; Cheng H.-M.; Polychronopoulou K. Design and synthesis of thermally stable single atom catalysts for thermochemical CO2 reduction. J. Energy Chem. 2023, 86, 246–262. 10.1016/j.jechem.2023.07.032. - DOI
LinkOut - more resources
Full Text Sources
