Efficacy and Safety of Lenvatinib Plus Programmed Death-1 Inhibitors with or Without Transarterial Chemoembolization in the Treatment of Unresectable Hepatocellular Carcinoma
- PMID: 39619601
- PMCID: PMC11606145
- DOI: 10.2147/JHC.S485047
Efficacy and Safety of Lenvatinib Plus Programmed Death-1 Inhibitors with or Without Transarterial Chemoembolization in the Treatment of Unresectable Hepatocellular Carcinoma
Abstract
Purpose: Transarterial chemoembolization (TACE) is recommended as a standard therapy for intermediate-stage hepatocellular carcinoma (HCC) and is the most widely used first-line treatment for advanced HCC. This study aimed to evaluate the clinical benefits and tolerability of TACE added to a combination of lenvatinib and programmed death-1 (PD-1) inhibitor in patients with unresectable HCC (uHCC).
Patients and methods: We conducted a retrospective cohort study involving 144 patients with uHCC treated between August 2020 and August 2023. Patients received a combination of lenvatinib and a PD-1 inhibitor with or without TACE (T+L+P, n=81 or L+P, n=63, respectively). The baseline characteristics of the two groups were compared, and propensity score matching (PSM) was used to minimize bias. The study endpoints included overall survival (OS), progression-free survival (PFS), and objective response rate (ORR). Factors influencing survival rates were analyzed using Cox regression, and adverse events (AEs) were documented and assessed.
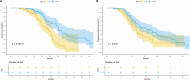
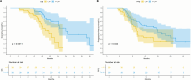
Results: Before PSM, the T+L+P group showed significantly higher ORR (64.1% vs 44.4%, p < 0.05), longer median PFS (14.3 vs 9.6 months, p < 0.05), and longer median OS (24.6 vs 19.5 months, p < 0.05) compared to the L+P group. Even post-PSM, the T+L+P group showed significantly better OS and PFS compared to the L+P group (mOS: 28.0 vs 17.6 months p=0.0011, mPFS: 15.8 vs 9.3 months, p < 0.05). Univariate and multivariate analyses identified treatment options as independent factors for PFS and OS. The safety profile of the T+L+P regimen was acceptableThe incidence and severity of adverse reactions in the T+L+P group were not significantly different compared to the L+P group (any grade, 90.1 vs 93.6%, p=0.551; grade≥3, 25.9 vs 23.8%, p=0.843).
Keywords: PD-1 inhibitor; combination therapy; lenvatinib; propensity score matching; transarterial chemoembolization; unresectable hepatocellular carcinoma.
© 2024 Jin et al.
Conflict of interest statement
The authors declare that there is no conflict of interest in the research and publication of this article.
Figures
References
LinkOut - more resources
Full Text Sources
Miscellaneous