Suppressed oncogenic molecules involved in the treatment of colorectal cancer by fecal microbiota transplantation
- PMID: 39619695
- PMCID: PMC11605715
- DOI: 10.3389/fmicb.2024.1451303
Suppressed oncogenic molecules involved in the treatment of colorectal cancer by fecal microbiota transplantation
Abstract
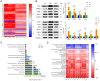
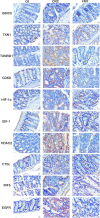
Dysbiosis of the intestinal microbiota is prevalent among patients with colorectal cancer (CRC). This study aims to explore the anticancer roles of the fecal microbiota in inhibiting the progression of colorectal cancer and possible mechanisms. The intestinal microbial dysbiosis in CRC mice was significantly ameliorated by fecal microbiota transplantation (FMT), as indicated by the restored ACE index and Shannon index. The diameter and number of cancerous foci were significantly decreased in CRC mice treated with FMT, along with the restoration of the intestinal mucosal structure and the lessening of the gland arrangement disorder. Key factors in oxidative stress (TXN1, TXNRD1, and HIF-1α); cell cycle regulators (IGF-1, BIRC5, CDK8, HDAC2, EGFR, and CTSL); and a critical transcription factor of the innate immune signal pathway (IRF5) were among the repressed oncogenic targets engaged in the FMT treatment of CRC. Correlation analysis revealed that their expressions were positively correlated with uncultured_bacterium_o_Mollicutes_RF39, Rikenellaceae_RC9_gut_group, and negatively correlated with Bacillus, Marvinbryantia, Roseburia, Angelakisella, Enterorhabdus, Bacteroides, Muribaculum, and genera of uncultured_bacterium_f_Eggerthellaceae, uncultured_bacterium_f_Xanthobacteraceae, Prevotellaceae_UCG-001, uncultured_bacterium_f_Erysipelotrichaceae, uncul-tured_bacterium_f_Lachnospiraceae, uncultured_bacterium_f_Ruminococcaceae, Eubacterium_coprostanoligenes_group, Ruminococcaceae_UCG-005, and uncultured_bacterium_f_Peptococcaceae. This study provides more evidence for the application of FMT in the clinical treatment of CRC.
Keywords: colorectal cancer; fecal microbiota transplantation; intestinal homeostasis; intestinal microbiota; oncogenic molecules.
Copyright © 2024 Han, Zhang, Zeng, Ma, Wang, Yuan, Xu, Geng, Fan, Gao, Arshad, Gao, Zhao, Liu and Mu.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures


References
-
- Chen W., Ye J., Tan X., Zhang Y., Liang J., Huang M. (2021). Analysis of 154 T4 colorectal cancer patients with peritoneal carcinomatosis treated by surgery. Int. J. Clin. Med. 12, 61–70. doi: 10.4236/ijcm.2021.122008 - DOI
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

