Mycosynthesis of silver nanoparticles from endophytic Aspergillus parasiticus and their antibacterial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo
- PMID: 39619697
- PMCID: PMC11604631
- DOI: 10.3389/fmicb.2024.1483637
Mycosynthesis of silver nanoparticles from endophytic Aspergillus parasiticus and their antibacterial activity against methicillin-resistant Staphylococcus aureus in vitro and in vivo
Abstract
Background: Methicillin-resistant Staphylococcus aureus (MRSA) is a drug-resistant and biofilm-forming pathogenic bacteria with severe morbidity and mortality. MRSA showed resistance against currently available antibiotics. Thus, there is an urgent need to develop novel effective treatments with minimal side effects to eliminate MRSA.
Aim: In this study, we aimed to mycosynthesize silver nanoparticles (AgNPs) using the endophytic fungus Aspergillus parasiticus isolated from leaves of Reseda Arabica and to examine their antibacterial activity against MRSA.
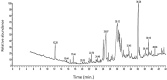
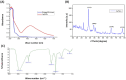
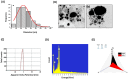
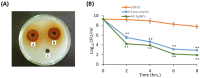
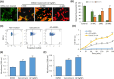
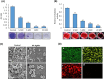
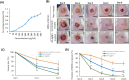
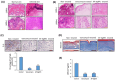
Results: Screening of fungal secondary metabolites using gas chromatography-mass spectroscopy (GC-MS) analysis revealed the presence of high content of bioactive compounds with antibacterial activities. AP-AgNPs were mycosynthesized for the first time using ethyl acetate extract of A. parasiticus and characterized by imaging (transmission electron microscopy (TEM), UV-Vis spectroscopy, zeta potential, X-ray diffraction (XRD), energy-dispersive X-ray analysis (EDX), and Fourier transform infrared spectroscopy (FTIR)). The agar well diffusion method revealed the antibacterial activity of AP-AgNPs against MRSA with 25 μg/mL of minimum inhibitory concentration (MIC). AP-AgNPs were shown to exert antibacterial action via a bactericidal mechanism based on flow cytometry, scanning electron microscopy, and transmission electron microscopy assessment. Our data demonstrated the effective interaction of AP-AgNPs with the bacterial cell membrane, which resulted in cell membrane damage and disruption of cell surface structure. Furthermore, AP-AgNPs successfully prevented the development of MRSA biofilms by disturbing cell adhesion and destructing mature biofilm reaching over 80% clearance rate. Interestingly, topical application of AP-AgNPs to superficial skin infection induced by MRSA in mice effectively promoted wound healing and suppressed bacterial burden.
Conclusion: Our results provide a novel green nanoparticle drug design with effective therapeutic potential against MRSA-induced skin infection.
Keywords: Aspergillus parasiticus; MRSA; silver nanoparticles; skin infection; wound healing.
Copyright © 2024 Ali, Rajendran and Abdallah.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. The author(s) declared that they were an editorial board member of Frontiers, at the time of submission. This had no impact on the peer review process and the final decision.
Figures

Similar articles
-
Critical Evaluation of Green Synthesized Silver Nanoparticles-Kaempferol for Antibacterial Activity Against Methicillin-Resistant Staphylococcus aureus.Int J Nanomedicine. 2024 Feb 8;19:1339-1350. doi: 10.2147/IJN.S431499. eCollection 2024. Int J Nanomedicine. 2024. PMID: 38348172 Free PMC article.
-
Antibacterial efficacy of silver nanoparticles and ethyl acetate's metabolites of the potent halophilic (marine) bacterium, Bacillus cereus A30 on multidrug resistant bacteria.Pathog Glob Health. 2017 Oct;111(7):367-382. doi: 10.1080/20477724.2017.1390829. Epub 2017 Oct 26. Pathog Glob Health. 2017. PMID: 29072532 Free PMC article.
-
Biogenic nanosilver bearing antimicrobial and antibiofilm activities and its potential for application in agriculture and industry.Front Microbiol. 2023 Feb 20;14:1125685. doi: 10.3389/fmicb.2023.1125685. eCollection 2023. Front Microbiol. 2023. PMID: 36891391 Free PMC article.
-
Biosynthesis of silver nanoparticles using endophytic Fusarium oxysporum strain NFW16 and their in vitro antibacterial potential.Microsc Res Tech. 2022 Apr;85(4):1568-1579. doi: 10.1002/jemt.24018. Epub 2021 Dec 9. Microsc Res Tech. 2022. PMID: 34888986
-
Nanoparticles in Antibacterial Therapy: A Systematic Review of Enhanced Efficacy against Intracellular Bacteria.ACS Omega. 2025 Apr 25;10(17):17070-17086. doi: 10.1021/acsomega.5c01813. eCollection 2025 May 6. ACS Omega. 2025. PMID: 40352514 Free PMC article. Review.
Cited by
-
Phytofabricated Farsetia aegyptia-derived silver nanoparticles mediate antibacterial and wound-healing activities in diabetic foot infection rat model.Inflammopharmacology. 2025 Jun;33(6):3233-3254. doi: 10.1007/s10787-025-01789-9. Epub 2025 Jun 3. Inflammopharmacology. 2025. PMID: 40461756
References
-
- Agoramoorthy G., Chandrasekaran M., Venkatesalu V., Hsu M. J. (2007). Antibacterial and antifungal activities of fatty acid methyl esters of the blind-your-eye mangrove from India. Braz. J. Microbiol. 38, 739–742. doi: 10.1590/S1517-83822007000400028 - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

