Effects of 30-Day Midodrine Administration, Compared to Placebo, on Blood Pressure, Cerebral Blood Flow Velocity, and Cognitive Performance in Persons with SCI
- PMID: 39619823
- PMCID: PMC11603111
- DOI: 10.46292/sci23-00038
Effects of 30-Day Midodrine Administration, Compared to Placebo, on Blood Pressure, Cerebral Blood Flow Velocity, and Cognitive Performance in Persons with SCI
Abstract
Background: Individuals with spinal cord injury (SCI) at and above T6 experience impaired descending cortical control of the autonomic nervous system, which predisposes them to blood pressure (BP) disorders including persistent hypotension.
Objectives: The primary aim of this investigation was to determine the effects of midodrine, 10 mg, administered daily over a 30-day period in the home environment, compared to placebo, on laboratory assessments of BP, cerebral blood flow velocity (CBFv), and cognitive performance in hypotensive individuals with chronic SCI.
Methods: This prospective, randomized, placebo-controlled, double-blind, crossover trial was conducted in 15 individuals with tetraplegia. In the first 30-day period, five participants were randomized to midodrine and 10 were randomized to placebo; participants were then crossed over to the second 30-day period following a 14-day washout. Laboratory assessments of BP, CBFv, and cognitive performance were measured before and after each of the two study arms.
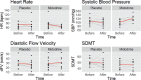
Results: Systolic BP (SBP) was significantly increased following midodrine administration compared to placebo (116 ± 23 mm Hg vs 94 ± 16 mm Hg; p = .002). In addition, diastolic CBFv was increased after midodrine administration compared to placebo (31.0 ± 11.2 vs 25.6 ± 9.1 cm/s; p = .04). However, there were no significant drug by time interaction effects for systolic or mean CBFv (p > .172) and cognitive performance (p = .689).
Conclusion: The results suggest significant increases in SBP and diastolic CBFv without appreciable effects on cognition after 30 days of midodrine administration. Further investigation is needed to identify effective antihypotensive treatment options that not only normalize BP but also improve CBFv and cognition.
Keywords: autonomic dysreflexia; hemodynamic instability; hypotension; orthostatic hypotension; spinal cord injuries; tetraplegia.
© 2024 American Spinal Injury Association.
Conflict of interest statement
Conflicts of Interest The authors declare no conflicts of interest.
Figures


References
-
- Wecht JM, Weir JP, Katzelnick CG, et al. Systemic and cerebral hemodynamic contribution to cognitive performance in spinal cord injury. J Neurotrauma. 2018;35(24):2957–2964. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
