Evaluation of mesenchymal stem cells as an in vitro model for inherited retinal diseases
- PMID: 39620144
- PMCID: PMC11604642
- DOI: 10.3389/fcell.2024.1455140
Evaluation of mesenchymal stem cells as an in vitro model for inherited retinal diseases
Abstract
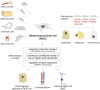
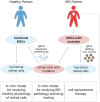
Retinal pathologies are major causes of vision impairment and blindness in humans, and inherited retinal diseases (IRDs), such as retinitis pigmentosa, Leber congenital amaurosis, and Stargardt disease, greatly contribute to this problem. In vitro disease modeling can be used for understanding the development of pathology and for screening therapeutic pharmaceutical compounds. In the preclinical research phase, in vitro models complement in vivo models by reducing animal studies, decreasing costs, and shortening research timelines. Additionally, animal models may not always accurately replicate the human disease phenotype. This review examines the types of cells that can be used to create in vitro IRD models, including retina-specific cell lines, primary retinal cells, induced pluripotent stem cells (iPSCs), and more. Special attention is given to mesenchymal stem cells (MSCs), which are characterized by various isolation sources, relative ease of isolation, and straightforward differentiation. MSCs derived from bone marrow (BM), adipose tissue (AT), dental tissue (DT), umbilical cord (UC), and other sources can differentiate into retinal cells, including photoreceptor cells and retinal pigment epithelial (RPE) cells, dysfunction of which is most commonly associated with IRDs. Subsequent differentiation of MSCs into retinal cells can be carried out via various methods: culturing in induction media supplemented with certain growth factors, co-culturing with retinal cells or in their conditioned media, or regulating gene expression with viral vector-delivered transcription factors (TFs) or microRNAs (miRNAs). Compared to the popular iPSCs, for example, MSC-based models are significantly cheaper and faster to obtain, making them more feasible for large-scale drug screening. Nevertheless, the existing differentiation methods need further optimization for this promising platform to receive the success it deserves.
Keywords: IRD; MSC; in vitro disease modeling; inherited retinal diseases; mesenchymal stem cells; retinal cells.
Copyright © 2024 Dodina, Gurtsieva, Karabelsky and Minskaia.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures



References
Publication types
LinkOut - more resources
Full Text Sources

