Single cell RNA-sequencing of feline peripheral immune cells with V(D)J repertoire and cross species analysis of T lymphocytes
- PMID: 39620216
- PMCID: PMC11604454
- DOI: 10.3389/fimmu.2024.1438004
Single cell RNA-sequencing of feline peripheral immune cells with V(D)J repertoire and cross species analysis of T lymphocytes
Abstract
Introduction: The domestic cat (Felis catus) is a valued companion animal and a model for virally induced cancers and immunodeficiencies. However, species-specific limitations such as a scarcity of immune cell markers constrain our ability to resolve immune cell subsets at sufficient detail. The goal of this study was to characterize circulating feline T cells and other leukocytes based on their transcriptomic landscape and T-cell receptor repertoire using single cell RNA-sequencing.
Methods: Peripheral blood from 4 healthy cats was enriched for T cells by flow cytometry cell sorting using a mouse anti-feline CD5 monoclonal antibody. Libraries for whole transcriptome, αβ T cell receptor transcripts and γδ T cell receptor transcripts were constructed using the 10x Genomics Chromium Next GEM Single Cell 5' reagent kit and the Chromium Single Cell V(D)J Enrichment Kit with custom reverse primers for the feline orthologs.
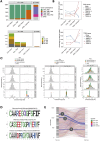
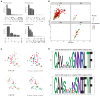
Results: Unsupervised clustering of whole transcriptome data revealed 7 major cell populations - T cells, neutrophils, monocytic cells, B cells, plasmacytoid dendritic cells, mast cells and platelets. Sub cluster analysis of T cells resolved naive (CD4+ and CD8+), CD4+ effector T cells, CD8+ cytotoxic T cells and γδ T cells. Cross species analysis revealed a high conservation of T cell subsets along an effector gradient with equitable representation of veterinary species (horse, dog, pig) and humans with the cat. Our V(D)J repertoire analysis identified a subset of CD8+ cytotoxic T cells with skewed TRA and TRB gene usage, conserved TRA and TRB junctional motifs, restricted TRA/TRB pairing and reduced diversity in TRG junctional length. We also identified a public γδ T cell subset with invariant TRD and TRG chains and a CD4+ TEM-like phenotype. Among monocytic cells, we resolved three clusters of classical monocytes with polarization into pro- and anti-inflammatory phenotypes in addition to a cluster of conventional dendritic cells. Lastly, our neutrophil sub clustering revealed a larger mature neutrophil cluster and a smaller exhausted/activated cluster.
Discussion: Our study is the first to characterize subsets of circulating T cells utilizing an integrative approach of single cell RNA-sequencing, V(D)J repertoire analysis and cross species analysis. In addition, we characterize the transcriptome of several myeloid cell subsets and demonstrate immune cell relatedness across different species.
Keywords: T cells; T-cell receptor repertoire; V(D)J; cross species analysis; feline; myeloid Cells; single cell RNA-sequencing (scRNA-seq).
Copyright © 2024 Ramarapu, Wulcan, Chang, Moore, Vernau and Keller.
Conflict of interest statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Figures







Update of
-
Single cell RNA-sequencing of feline peripheral immune cells with V(D)J repertoire and cross species analysis of T lymphocytes.bioRxiv [Preprint]. 2024 May 23:2024.05.21.595010. doi: 10.1101/2024.05.21.595010. bioRxiv. 2024. Update in: Front Immunol. 2024 Nov 15;15:1438004. doi: 10.3389/fimmu.2024.1438004. PMID: 38826195 Free PMC article. Updated. Preprint.
References
-
- Rowan AN. Companion Animal Statistics in the USA. (2018). Available online at: https://www.wellbeingintlstudiesrepository.org/demscapop/7/. (Accessed February 20, 2024).
-
- Larkin M, Radich R, AVMA. Am Vet Med Assoc . Pet population still on the rise, with fewer pets per household (2021). Available online at: https://avmajournals.avma.org/display/post/news/pet-population-still-on-.... (Accessed February 20, 2024).
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

