Development of Broad-Spectrum β-Cyclodextrins-Based Nanomaterials Against Influenza Viruses
- PMID: 39620346
- PMCID: PMC11609907
- DOI: 10.1002/jmv.70101
Development of Broad-Spectrum β-Cyclodextrins-Based Nanomaterials Against Influenza Viruses
Abstract
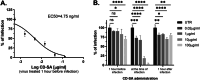
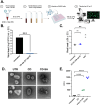
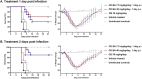
In recent decades, epidemics and pandemics have multiplied throughout the world, with viruses generally being the primary responsible agents. Among these, influenza viruses play a key role, as they potentially cause severe respiratory distress, representing a major threat to public health. Our study aims to develop new broad-spectrum antivirals against influenza to improve the response to viral disease outbreaks. We engineered macromolecules (named CD-SA) consisting of a β-cyclodextrin scaffold modified with hydrophobic linkers in the primary face, onto which unitary sialic acid epitopes are covalently grafted to mimic influenza virus-host receptors. We assessed the antiviral efficacy, mechanism of action, and the genetic barrier to resistance of this compound against influenza in vitro, ex vivo, and in vivo. We demonstrated that CD-SA, with a unitary SA, without extensive polysaccharides or specific connectivity, acts as a potent virucidal antiviral against several human influenza A and B viruses. Additionally, CD-SA displayed antiviral activity against SARS-CoV-2, a virus that also relies on sialic acid for attachment. We then assessed the genetic barrier to resistance for CD-SA. While resistance emerged after six passages with CD-SA alone, the virus remained sensitive through eight passages when co-treated with interferon-λ1 (IFN λ1). Finally, we completed the characterization of the antiviral activity by conducting both ex vivo and in vivo studies, demonstrating a potent antiviral effect in human airway epithelia and in a mouse model of infection, higher than that of Oseltamivir, a currently approved anti-influenza antiviral. The findings presented in this study support the potential therapeutic utility of a novel β-cyclodextrin-based nanomaterial for the treatment of influenza infections and potentially other sialic acid-dependent viruses.
Keywords: antiviral; broad‐spectrum; influenza; nanomaterials; virucidal; β‐cyclodextrins.
© 2024 The Author(s). Journal of Medical Virology published by Wiley Periodicals LLC.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

Similar articles
-
Inhibition of influenza A virus and SARS-CoV-2 infection or co-infection by griffithsin and griffithsin-based bivalent entry inhibitor.mBio. 2024 May 8;15(5):e0074124. doi: 10.1128/mbio.00741-24. Epub 2024 Apr 9. mBio. 2024. PMID: 38587427 Free PMC article.
-
Influenza A(H1N1)pdm09 virus resistance to baloxavir, oseltamivir and sialic acid mimetics in single and dual therapies: Insights from human airway epithelia and murine models.Antiviral Res. 2025 Jul;239:106174. doi: 10.1016/j.antiviral.2025.106174. Epub 2025 May 3. Antiviral Res. 2025. PMID: 40324597
-
Synergistic effects of Lianhuaqingwen in combination with Oseltamivir and Baloxavir against seasonal influenza virus: In vitro and in vivo assessment.J Ethnopharmacol. 2025 Feb 10;338(Pt 2):119091. doi: 10.1016/j.jep.2024.119091. Epub 2024 Nov 9. J Ethnopharmacol. 2025. PMID: 39528119
-
Antiviral strategies against influenza virus: an update on approved and innovative therapeutic approaches.Cell Mol Life Sci. 2025 Feb 13;82(1):75. doi: 10.1007/s00018-025-05611-1. Cell Mol Life Sci. 2025. PMID: 39945883 Free PMC article. Review.
-
Antiviral agents and therapeutics against respiratory viruses.Expert Opin Investig Drugs. 2024 Nov;33(11):1129-1133. doi: 10.1080/13543784.2024.2401911. Epub 2024 Sep 8. Expert Opin Investig Drugs. 2024. PMID: 39245955 Review.
Cited by
-
A pan-respiratory virus attachment inhibitor with high potency in human airway models and in vivo.Sci Adv. 2025 Aug;11(31):eadv9311. doi: 10.1126/sciadv.adv9311. Epub 2025 Aug 1. Sci Adv. 2025. PMID: 40749064 Free PMC article.
References
-
- Knobler S., Mahmoud A., Lemon S., and Pray L., eds., The Impact of Globalization on Infectious Disease Emergence and Control: Exploring the Consequences and Opportunities: Workshop Summary (Washington DC: National Academies Press, 2006). - PubMed
-
- Long J. S., Mistry B., Haslam S. M., and Barclay W. S., “Host and Viral Determinants of Influenza A Virus Species Specificity,” Nature Reviews Microbiology 17, no. 2 (2019): 67–81. - PubMed
-
- Tavares L. P., Teixeira M. M., and Garcia C. C., “The Inflammatory Response Triggered by Influenza Virus: A Two Edged Sword,” Inflammation Research 66, no. 4 (2017): 283–302. - PubMed
MeSH terms
Substances
Grants and funding
- HHSN272201700041I/AI/NIAID NIH HHS/United States
- This research was funded by the University of Geneva, Swiss National Science Foundation, Switzerland) (grant number Sinergia CRSII5_180323) to C.T., and partly by (grant number 310030_207418) to T.R. and by funding from the National Institutes of Health (grant number HHSN272201700041I/75N93021F00227).
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

