Design and Characterization of a Novel Intravitreal Dual-Transgene Genetic Medicine for Neovascular Retinopathies
- PMID: 39620832
- PMCID: PMC11614000
- DOI: 10.1167/iovs.65.14.1
Design and Characterization of a Novel Intravitreal Dual-Transgene Genetic Medicine for Neovascular Retinopathies
Abstract
Purpose: Intravitreal delivery of therapeutic transgenes to the retina via engineered viral vectors can provide sustained local concentrations of therapeutic proteins and thus potentially reduce the treatment burden and improve long-term vision outcomes for patients with neovascular (wet) age-related macular degeneration (AMD), diabetic macular edema (DME), and diabetic retinopathy.
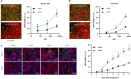
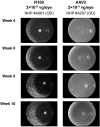
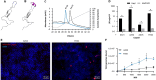
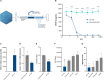
Methods: We performed directed evolution in nonhuman primates (NHP) to invent an adeno-associated viral (AAV) variant (R100) with the capacity to cross vitreoretinal barriers and transduce all regions and layers of the retina following intravitreal injection. We then engineered 4D-150, an R100-based genetic medicine carrying 2 therapeutic transgenes: a codon-optimized sequence encoding aflibercept, a recombinant protein that inhibits VEGF-A, VEGF-B, and PlGF, and a microRNA sequence that inhibits expression of VEGF-C. Transduction, transgene expression, and biological activity were characterized in human retinal cells in vitro and in NHPs.
Results: R100 demonstrated superior retinal cell transduction in vitro and in vivo compared to AAV2, a commonly used wild-type AAV serotype in retinal gene therapies. Transduction of human retinal pigment epithelial cells in vitro by 4D-150 resulted in dose-dependent transgene expression and corresponding reductions in VEGF-A and VEGF-C. Intravitreal administration of 4D-150 to NHPs was well tolerated and led to robust retinal expression of both transgenes. In a primate model of laser-induced choroidal neovascularization, 4D-150 completely prevented clinically relevant angiogenic lesions at all tested doses.
Conclusions: These findings support further development of 4D-150. Clinical trials are underway to establish the safety and efficacy of 4D-150 in individuals with wet AMD and DME.
Conflict of interest statement
Disclosure:
Figures


References
-
- Jonas JB, Cheung CMG, Panda-Jonas S.. Updates on the epidemiology of age-related macular degeneration. Asia Pac J Ophthalmol (Phila) . 2017; 6: 493–497. - PubMed
-
- Wong WL, Su X, Li X, et al. .. Global prevalence of age-related macular degeneration and disease burden projection for 2020 and 2040: a systematic review and meta-analysis. Lancet Glob Health . 2014; 2: e106–e116. - PubMed
-
- Hobbs SD, Pierce K.. Wet age-related macular degeneration (Wet AMD). StatPearls . Treasure Island (FL): StatPearls Publishing; 2022. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

