Comparison of On-Label Treatment Persistence in Real-World Patients with Psoriatic Arthritis Receiving Guselkumab Versus Subcutaneous Interleukin-17A Inhibitors
- PMID: 39621228
- PMCID: PMC11787232
- DOI: 10.1007/s12325-024-03042-1
Comparison of On-Label Treatment Persistence in Real-World Patients with Psoriatic Arthritis Receiving Guselkumab Versus Subcutaneous Interleukin-17A Inhibitors
Abstract
Introduction: Psoriatic arthritis (PsA) is a chronic, multidomain, inflammatory disease requiring long-term treatment. Guselkumab, a fully human interleukin [IL]-23p19-subunit inhibitor, and the IL-17A inhibitors (IL-17Ai) ixekizumab and secukinumab are approved by the US Food and Drug Administration (FDA) for adults with active PsA. Real-world data evaluating on-label treatment persistence is an important consideration for patients.
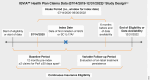
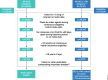
Methods: This retrospective claim-based analysis (IQVIA PharMetrics® Plus) included adults with PsA receiving guselkumab or their first subcutaneous (SC) IL-17Ai (ixekizumab/secukinumab) per FDA label ("on-label") between July 14, 2020, and June 30, 2022. Baseline demographic and disease characteristics were collected in the 12 months preceding the index date (date of first guselkumab or SC IL-17Ai claim); follow-up extended through the earlier of the end of continuous insurance eligibility or end of data availability. Baseline characteristics were balanced between the cohorts by propensity score weighting (standardized mortality ratio [SMR]). Discontinuation was defined as a gap 2 × the FDA-approved maintenance dosing interval (guselkumab:112 days; SC IL-17Ai: 56 days); on-label persistence in the weighted cohorts was assessed using Kaplan-Meier curves and compared with a Cox proportional hazards model.
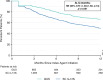
Results: Weighted demographic and disease characteristics were well balanced between the cohorts (guselkumab: N = 910, mean age = 50.4 years, 60.4% female; SC IL-17Ai: N = 2740, mean age = 50.2, 59.4% female). At 12 months, the guselkumab cohort was 1.85 × more likely to remain persistent with on-label therapy vs the SC IL-17Ai cohort (p < 0.001); median time to discontinuation was not reached for guselkumab and was 12.3 months for SC IL-17Ai. At 3, 6, 9, and 12 months, persistence rates in the weighted cohorts were higher with guselkumab than with SC IL-17Ai (p < 0.001).
Conclusion: In this real-world claims data analysis in adults with PsA, on-label persistence rates were statistically significantly higher with guselkumab, as early as 3 months, with ~ 2 × greater likelihood of persistence at 12 months relative to SC IL-17Ai.
Keywords: Guselkumab; Persistence; Psoriatic arthritis; Real-world evidence; Subcutaneous interleukin-17A inhibitors.
Plain language summary
Psoriatic arthritis is a chronic, progressive, inflammatory disease that requires long-term treatment. Overproduction of proteins called cytokines, including interleukin (IL)-23 and IL-17A, are known to be involved in psoriatic arthritis. Current treatment guidelines for patients with psoriatic arthritis recommend using medications made from antibodies (biologics), including those that inhibit IL-23 and IL-17A, for some patients. Guselkumab is an antibody medication that blocks IL-23; ixekizumab and secukinumab are antibody medications that block IL-17A. All three treatments are approved by the United States Food and Drug Administration for the treatment of psoriatic arthritis and are given subcutaneously at specific dosing regimens (referred to as “on-label”). This study used information collected from health insurance prescription claims to measure how many patients who started taking guselkumab, ixekizumab, or secukinumab using an on-label dosage were still taking this medication after 12 months. Anonymous patient information was selected from the database for adults who had at least two claims for psoriatic arthritis and started taking either guselkumab or one of the two IL-17A blockers (ixekizumab, secukinumab) between July 14, 2020 and June 30, 2022. Medication claims were included from 910 patients taking guselkumab and 2740 patients taking an IL-17 blocker. The characteristics of these groups (e.g., age, comorbidities, prior psoriatic arthritis treatments) were balanced between the two groups using a statistical method called propensity score weighting. At 1 year, patients in the guselkumab group were almost twice as likely to still be using their medication as the patients using ixekizumab or secukinumab.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Conflict of interest: Philip J Mease has received consulting fees from AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Immagene, Janssen, Novartis, Pfizer, UCB, and Ventyx; speaker fees from AbbVie, Amgen, Eli Lilly, Janssen, Novartis, Pfizer, and UCB; and research grants from AbbVie, Acelyrin, Amgen, Bristol Myers Squibb, Eli Lilly, Janssen, Novartis, and UCB. Shannon A Ferrante, Natalie J Shiff, Timothy P Fitzgerald, and Soumya D Chakravarty are or were employees of Janssen Scientific Affairs, LLC, a Johnson & Johnson company at the time this work was performed and own stock in Johnson & Johnson. Natalie J Shiff also owns stock in or has owned stock in AbbVie, Gilead, Iovance, Novo-Nordisk, and Pfizer within the past 3 years and is currently an employee of Alpine Immune Sciences, A Vertex Company. Jessica A Walsh has received consulting fees for AbbVie, Eli Lilly, Janssen, Novartis, and UCB; and research funding from AbbVie, Merck, and Pfizer. Ethical approval: The IQVIA PharMetrics® Plus database, used under license for this study, complies with Health Insurance Portability and Accountability Act (HIPAA) regulations. The data obtained from these analyses were de-identified; thus, no Institutional Review Board approval was required.
Figures
References
-
- Ritchlin CT, Colbert RA, Gladman DD. Psoriatic arthritis. N Engl J Med. 2017;376:957–70. - PubMed
-
- Tremfya: Package insert. Horsham: Janssen Biotech, Inc.; 2024.
-
- Deodhar A, Helliwell PS, Boehncke WH, et al. Guselkumab in patients with active psoriatic arthritis who were biologic-naive or had previously received TNFα inhibitor treatment (DISCOVER-1): a double-blind, randomised, placebo-controlled phase 3 trial. Lancet. 2020;395:1115–25. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

