Ticagrelor vs Prasugrel for Acute Coronary Syndrome in Routine Care
- PMID: 39621344
- PMCID: PMC11612834
- DOI: 10.1001/jamanetworkopen.2024.48389
Ticagrelor vs Prasugrel for Acute Coronary Syndrome in Routine Care
Erratum in
-
Errors in the Results, Table 2, Figures 2 and 3, and Supplement 1.JAMA Netw Open. 2025 Jan 2;8(1):e2460952. doi: 10.1001/jamanetworkopen.2024.60952. JAMA Netw Open. 2025. PMID: 39821403 Free PMC article. No abstract available.
Abstract
Importance: In patients with acute coronary syndrome (ACS) undergoing invasive treatment, ticagrelor and prasugrel are guideline-recommended P2Y12 receptor inhibitors. The ISAR-REACT5 randomized clinical trial demonstrated superiority for prasugrel, although concerns were raised about the generalizability of some underpowered subgroup analyses.
Objectives: To emulate a randomized clinical trial evaluating the safety and effectiveness of ticagrelor vs prasugrel under the conditions of routine care in individuals with ACS planned to undergo an invasive treatment strategy.
Design, setting, and participants: This new-user cohort study included secondary data from a German statutory health insurance claims database between January 2012 and December 2021, using 1:1 propensity score nearest-neighbor matching to emulate ISAR-REACT5. Individuals with ACS receiving either ticagrelor or prasugrel treatment after hospital discharge were followed up for 1 year. Eligibility criteria closely emulated those of ISAR-REACT5 and included age of 18 years or older and cardiovascular risk factors. Data were analyzed from May 2023 to May 2024.
Exposure: Outpatient prescription of ticagrelor or prasugrel.
Main outcomes and measures: The primary end point was the composite of all-cause mortality, myocardial infarction (MI), or stroke within 1 year of outpatient treatment initiation. Secondary end points included individual components of the primary end point and stent thrombosis. The safety end point was major bleeding. A Cox proportional hazards regression model was fitted to the overall cohort.
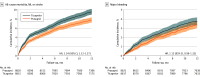
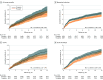
Results: Of 17 642 propensity score-matched individuals (mean [SD] age, 63.1 [10.9] years; 73.9% male), 8821 received ticagrelor and 8821 received prasugrel. Agreement was met in 11 of 12 predefined agreement metrics when comparing the results with ISAR-REACT5. The primary composite end point of all-cause mortality, MI, or stroke occurred in 815 individuals (9.2%) receiving ticagrelor and 663 (7.5%) receiving prasugrel (hazard ratio [HR], 1.24; 95% CI, 1.12-1.37). Myocardial infarction (HR, 1.20; 95% CI, 1.06-1.36) and stroke (HR, 1.33; 95% CI, 1.02-1.74) each occurred significantly more often in the ticagrelor group. Analysis of all-cause mortality (HR, 1.27; 95% CI, 0.99-1.64), stent thrombosis (HR, 1.11; 95% CI, 0.89-1.30), and major bleeding (HR, 1.12; 95% CI, 0.96-1.32) revealed no significant differences between treatment groups. Subgroup analysis showed that prasugrel was associated with the primary composite end point in fewer individuals with ST-segment elevation MI (338 of 4941 [6.8%] vs 451 of 4852 [9.3%]).
Conclusions and relevance: This cohort study found that prasugrel was associated with lower rates of all-cause mortality, MI, or stroke compared with ticagrelor in individuals with ACS undergoing an invasive treatment strategy in routine care, particularly in individuals with ST-segment elevation MI. The findings suggest that carefully designed database studies can complement and extend findings from randomized clinical trials, informing guidelines and clinical decision-making.
Conflict of interest statement
Figures

References
-
- Motovska Z, Hlinomaz O, Miklik R, et al. ; PRAGUE-18 Study Group . Prasugrel versus ticagrelor in patients with acute myocardial infarction treated with primary percutaneous coronary intervention: multicenter randomized PRAGUE-18 study. Circulation. 2016;134(21):1603-1612. doi:10.1161/CIRCULATIONAHA.116.024823 - DOI - PubMed
-
- Levine GN, Bates ER, Bittl JA, et al. . 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary intervention, 2011 ACCF/AHA guideline for coronary artery bypass graft surgery, 2012 ACC/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease, 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction, 2014 AHA/ACC guideline for the management of patients with non-ST-elevation acute coronary syndromes, and 2014 ACC/AHA guideline on perioperative cardiovascular evaluation and management of patients undergoing noncardiac surgery. Circulation. 2016;134(10):e123-e155. doi:10.1161/CIR.0000000000000404 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

