Postischemic inactivation of HIF prolyl hydroxylases in endothelium promotes maladaptive kidney repair by inducing glycolysis
- PMID: 39621585
- PMCID: PMC11785929
- DOI: 10.1172/JCI176207
Postischemic inactivation of HIF prolyl hydroxylases in endothelium promotes maladaptive kidney repair by inducing glycolysis
Abstract
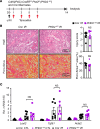
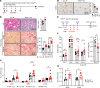
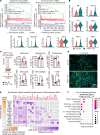
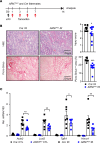
Ischemic acute kidney injury (AKI) is common in hospitalized patients and increases the risk for chronic kidney disease (CKD). Impaired endothelial cell (EC) functions are thought to contribute in AKI to CKD transition, but the underlying mechanisms remain unclear. Here, we identify a critical role for endothelial oxygen sensing prolyl hydroxylase domain (PHD) enzymes 1-3 in regulating postischemic kidney repair. In renal endothelium, we observed compartment-specific differences in the expression of the 3 PHD isoforms in both mice and humans. Postischemic concurrent inactivation of endothelial PHD1, PHD2, and PHD3 but not PHD2 alone promoted maladaptive kidney repair characterized by exacerbated tissue injury, fibrosis, and inflammation. scRNA-Seq analysis of the postischemic endothelial PHD1, PHD2, and PHD3-deficient (PHDTiEC) kidney revealed an endothelial hypoxia and glycolysis-related gene signature, also observed in human kidneys with severe AKI. This metabolic program was coupled to upregulation of the SLC16A3 gene encoding the lactate exporter monocarboxylate transporter 4 (MCT4). Strikingly, treatment with the MCT4 inhibitor syrosingopine restored adaptive kidney repair in PHDTiEC mice. Mechanistically, MCT4 inhibition suppressed proinflammatory EC activation, reducing monocyte-EC interaction. Our findings suggest avenues for halting AKI to CKD transition based on selectively targeting the endothelial hypoxia-driven glycolysis/MCT4 axis.
Keywords: Chronic kidney disease; Endothelial cells; Hypoxia; Metabolism; Nephrology.
Conflict of interest statement
Figures




Update of
-
Post-ischemic inactivation of HIF prolyl hydroxylases in endothelium promotes maladaptive kidney repair by inducing glycolysis.bioRxiv [Preprint]. 2023 Oct 3:2023.10.03.560700. doi: 10.1101/2023.10.03.560700. bioRxiv. 2023. Update in: J Clin Invest. 2024 Dec 02;135(3):e176207. doi: 10.1172/JCI176207. PMID: 37873349 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

