The NF-κB-HE4 axis: A novel regulator of HE4 secretion in ovarian cancer
- PMID: 39621651
- PMCID: PMC11611113
- DOI: 10.1371/journal.pone.0314564
The NF-κB-HE4 axis: A novel regulator of HE4 secretion in ovarian cancer
Abstract
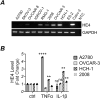
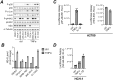
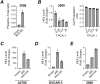
Ovarian cancer is the leading cause of death among gynecologic malignancies. Despite recent advancements in targeted therapies such as PARP inhibitors, recurrence is common and frequently resistant to existing therapies. A powerful diagnostic tool, coupled with a comprehensive understanding of its implications, is crucial. HE4, a clinical serum biomarker for ovarian cancer, has shown efficacy in monitoring malignant phenotypes, yet little is known about its biological role and regulatory mechanisms. Our research demonstrates that HE4 expression in ovarian cancer can be regulated by the NF-κB signaling pathway. We found that the activation of NF-κB signaling by tumor necrosis factor (TNF)-α, a cytokine found in ovarian cancer tumors and ascites, enhanced the secretion of HE4 while its inhibition suppressed HE4 levels. Nuclear translocation of the NF-κB component p65 was found to be critical for HE4 expression; induced NF-κB activation through p65 expression or constitutive IKK2 activity elevated HE4 expression, while p65 knockdown had the opposite effect. Furthermore, we observed that NF-κB mediated HE4 expression at the transcriptional level. Our data also suggests that there is a regulatory role for HE4 in the expression of α5-Integrin, a crucial adhesion molecule in ovarian cancer metastasis; HE4 knockdown corresponded with reduced α5-Integrin expression, cell migration and cell adhesion to fibronectin.
Copyright: © 2024 Kim et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures



References
-
- SEER Cancer Stat Facts: Ovarian Cancer. National Cancer Institute. Bethesda M, https://seer.cancer.gov/statfacts/html/ovary.html.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials

