Amino Acid and Glucose Fermentation Maintain ATP Content in Mouse and Human Malignant Glioma Cells
- PMID: 39621724
- PMCID: PMC11792161
- DOI: 10.1080/17590914.2024.2422268
Amino Acid and Glucose Fermentation Maintain ATP Content in Mouse and Human Malignant Glioma Cells
Abstract
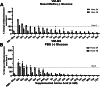
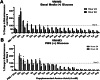
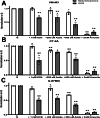
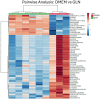
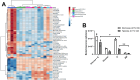
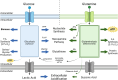
Energy is necessary for tumor cell viability and growth. Aerobic glucose-driven lactic acid fermentation is a common metabolic phenotype seen in most cancers including malignant gliomas. This metabolic phenotype is linked to abnormalities in mitochondrial structure and function. A luciferin-luciferase bioluminescence ATP assay was used to measure the influence of amino acids, glucose, and oxygen on ATP content and viability in mouse (VM-M3 and CT-2A) and human (U-87MG) glioma cells that differed in cell biology, genetic background, and species origin. Oxygen consumption was measured using the Resipher system. Extracellular lactate and succinate were measured as end products of the glycolysis and glutaminolysis pathways, respectively. The results showed that: (1) glutamine was a source of ATP content irrespective of oxygen. No other amino acid could replace glutamine in sustaining ATP content and viability; (2) ATP content persisted in the absence of glucose and under hypoxia, ruling out substantial contribution through either glycolysis or oxidative phosphorylation (OxPhos) under these conditions; (3) Mitochondrial complex IV inhibition showed that oxygen consumption was not an accurate measure for ATP production through OxPhos. The glutaminase inhibitor, 6-diazo-5-oxo-L-norleucine (DON), reduced ATP content and succinate export in cells grown in glutamine. The data suggests that mitochondrial substrate level phosphorylation in the glutamine-driven glutaminolysis pathway contributes to ATP content in these glioma cells. A new model is presented highlighting the synergistic interaction between the high-throughput glycolysis and glutaminolysis pathways that drive malignant glioma growth and maintain ATP content through the aerobic fermentation of both glucose and glutamine.
Keywords: Fermentation; glioblastoma; glutaminolysis; mitochondrial substrate level phosphorylation; succinate.
Plain language summary
Malignant gliomas, regardless of cell of origin or species, rely on fermentation mechanisms for ATP production due to OxPhos insufficiency. Glucose and glutamine together are necessary and sufficient for dysregulated tumor cell growth, whereas OxPhos is neither necessary nor sufficient.
Figures


References
-
- Aisenberg, A. C. (1961). The glycolysis and respiration of tumors. Academic Press.
-
- Arcos, J. C., Tison, M. J., Gosch, H. H., & Fabian, J. A. (1969). Sequential alterations in mitochondrial inner and outer membrane electron transport and in respiratory control during feeding of amino azo dyes; stability of phosphorylation. Correlation with swelling-contraction changes and tumorigenesis threshold. Cancer Research, 29(6), 1298–1305. - PubMed
-
- Astiarraga, B., Martínez, L., Ceperuelo-Mallafré, V., Llauradó, G., Terrón-Puig, M., Rodríguez, M. M., Casajoana, A., Pellitero, S., Megía, A., Vilarrasa, N., Vendrell, J., & Fernández-Veledo, S. (2020). Impaired succinate response to a mixed meal in obesity and type 2 diabetes is normalized after metabolic surgery. Diabetes Care, 43(10), 2581–2587. 10.2337/dc20-0460 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
