Melanocortin 1 receptor mediates melanin production by interacting with the BBSome in primary cilia
- PMID: 39621784
- PMCID: PMC11637432
- DOI: 10.1371/journal.pbio.3002940
Melanocortin 1 receptor mediates melanin production by interacting with the BBSome in primary cilia
Abstract
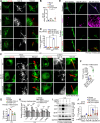
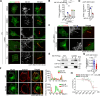
Production of melanin pigments is a protective mechanism of the skin against ultraviolet (UV)-induced damage and carcinogenesis. However, the molecular basis for melanogenesis is still poorly understood. Herein, we demonstrate a critical interplay between the primary cilium and the melanocortin 1 receptor (MC1R) signaling. Our data show that UV and α-melanocyte-stimulating hormone (α-MSH) trigger cilium formation in human melanocytes and melanoma cells. Deficiency of MC1R or the presence of its red hair color (RHC) variations significantly attenuates the UV/α-MSH-induced ciliogenesis. Further investigation reveals that MC1R enters the cilium upon UV/α-MSH stimulation, which is facilitated by the interaction of MC1R with the BBSome and the palmitoylation of MC1R. MC1R interacts with the BBSome through the second and third intercellular loops, which contain the common RHC variant alleles (R151C and R160W). These RHC variants of MC1R exhibit attenuated ciliary localization, and enforced ciliary localization of these variants elevates melanogenesis. Ciliary MC1R triggers a sustained cAMP signaling and selectively stimulates Sox9, which appears to up-regulate melanogenesis-related genes as the transcriptional cofactor for MITF. These findings reveal a previously unrecognized nexus between MC1R and cilia and suggest an important mechanism for RHC variant-related pigmentary defects.
Copyright: © 2024 Tian et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

