Interferon epsilon is produced in the testis and protects the male reproductive tract against virus infection, inflammation and damage
- PMID: 39621805
- PMCID: PMC11637430
- DOI: 10.1371/journal.ppat.1012702
Interferon epsilon is produced in the testis and protects the male reproductive tract against virus infection, inflammation and damage
Abstract
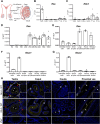
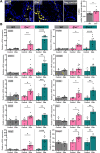
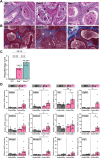
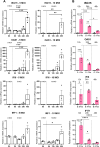
The testis is a reservoir for viruses that can cause persistent infection and adversely affect male reproductive health, an observation commonly attributed to deficiencies in inducible antiviral defence mechanisms. In this study, we demonstrate that interferon-epsilon (IFNε), a type I interferon initially discovered in female reproductive epithelia, is constitutively expressed by meiotic and post-meiotic spermatogenic cells, Leydig cells and macrophages in mouse testes. A similar distribution pattern was observed in human testes. Mice lacking IFNɛ were more susceptible to Zika virus-induced inflammation and damage of the testis and epididymis compared to wild-type mice. Exogenous IFNε treatment reduced the viral infection burden in cultured human testicular cells by inducing interferon-stimulated gene expression, and reducing inflammatory gene expression and cell damage. Treatment was more effective when administered prior to infection. These data indicate a critical role for constitutively-expressed IFNɛ in limiting viral infection and inflammatory damage in the male reproductive tract.
Copyright: © 2024 Wijayarathna et al. This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits unrestricted use, distribution, and reproduction in any medium, provided the original author and source are credited.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures




References
-
- Pilatz A, Arneth B, Kaiser R, Heger E, Pirkl M, Böttcher S, Fritzenwanker M, Renz H, Mankertz A, Schuppe HC, Wagenlehner F. Acute orchitis deciphered: Coxsackievirus B strains are the main etiology and their presence in semen is associated with acute inflammation and risk of persistent oligozoospermia. J Med Virol (2023). 95, e28970. doi: 10.1002/jmv.28970 - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

