How do we transfuse children in Italy? Results of a national survey
- PMID: 39621895
- PMCID: PMC11925250
- DOI: 10.2450/BloodTransfus.838
How do we transfuse children in Italy? Results of a national survey
Abstract
Background: Adherence to optimal practices in the preparation and issuance of pediatric blood components can significantly influence patient care outcomes. This study aims to examine the blood banking procedures across prominent Italian children's hospitals, with the goal of identifying both consistent and potentially divergent standards within this field.
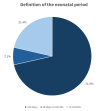
Materials and methods: A survey was conducted among the blood banks affiliated with the Italian Association of Pediatric Hospitals. Modeled after the AABB Neonatal and Pediatric Blood Bank Practices Survey, the questionnaire comprised 25 questions covering hospital characteristics, definitions of the neonatal period, pre-transfusion tests, blood component availability, and irradiation protocols.
Results: Fourteen out of the sixteen invited blood banks participated in the survey. The findings revealed a wide range of practices among the surveyed hospitals. Major differences were noted in the neonatal period definition, pre-transfusion compatibility procedures, and platelet transfusion protocols. All hospitals provided leukodepleted packed red blood cells (pRBCs), with differences in availability of autologous blood and reconstituted whole blood. Irradiated blood components were universally accessible, with differences in post-irradiation acceptable storage time. Additionally, differences in dosages for packed red blood cells (pRBCs) and platelet concentrates (PCs) were observed across hospitals.
Conclusions: Standardized guidelines for pediatric transfusion practices within Italian blood banks are of paramount importance. The observed variability underscores the necessity for sharing best practices among centers supplying blood components to pediatric patients.
Conflict of interest statement
The Authors declare no conflict of interest.
Figures




References
-
- World Health Organization. Model list of essential medicines - 21st List 2019. [Accessed on 07/04/2024]. Available at: https://www.who.int/publications/i/item/WHOMVPEMPIAU2019.06.
-
- Bateman ST, Lacroix J, Boven K, Forbes P, Barton R, Thomas NJ, et al. Pediatric acute lung injury and sepsis investigators network. Anemia, blood loss, and blood transfusions in North American children in the intensive care unit. Am J Respir Crit Care Med. 2008;178:26–33. doi: 10.1164/rccm.200711-1637OC. - DOI - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
