Patient-Relevant Digital-Motor Outcomes for Clinical Trials in Hereditary Spastic Paraplegia Type 7: A Multicenter PROSPAX Study
- PMID: 39621946
- PMCID: PMC11606240
- DOI: 10.1212/WNL.0000000000209887
Patient-Relevant Digital-Motor Outcomes for Clinical Trials in Hereditary Spastic Paraplegia Type 7: A Multicenter PROSPAX Study
Abstract
Background and objectives: With targeted treatment trials on the horizon, identification of sensitive and valid outcome measures becomes a priority for >100 spastic ataxias. While digital-motor measures, assessed using wearable sensors, are considered prime outcome candidates for spastic ataxias, genotype-specific validation studies are lacking. We here aimed to identify candidate digital-motor outcomes for spastic paraplegia type 7 (SPG7)-one of the most common spastic ataxias-that (1) reflect patient-relevant health aspects, even in mild, trial-relevant disease stages; (2) are suitable for a multicenter setting; and (3) assess mobility also during uninstructed walking simulating real life.
Methods: This cross-sectional multicenter study (7 centers, 6 countries) analyzed defined laboratory-based walking and uninstructed "supervised free walking" in patients with SPG7 and healthy controls using 3 wearable sensors (Opal APDM). For the extracted digital gait measures, we assessed effect sizes for the discrimination of patients and controls (Cliff δ) and Spearman correlations with measures of functional mobility and overall disease severity (Spastic Paraplegia Rating Scale [SPRS], including mobility subscore SPRSmobility; Scale for the Assessment and Rating of Ataxia [SARA]) and the activities of daily living subscore of the Friedreich Ataxia Rating Scale (FARS-ADL).
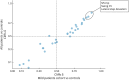
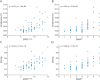
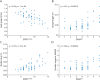
Results: Gait was analyzed in 65 patients with SPG7 and 50 healthy controls. Among 30 hypothesis-based gait measures, 18 demonstrated at least moderate effect size (δ > 0.5) in discriminating patients from controls and 17 even in mild disease stages (SPRSmobility ≤ 9, n = 41). Spatiotemporal variability measures such as spatial variability measure SPcmp (ρ = 0.67, p < 0.0001) and stride time CV (ρ = 0.67, p < 0.0001) showed the largest correlations with functional mobility (SPRSmobility)-as with overall disease severity (SPRS, SARA) and activities of daily living (FARS-ADL). The correlations of variability measures with SPRSmobility could be confirmed in mild disease stages (e.g., SPcmp: ρ = 0.50, p < 0.0001) and in "supervised free walking" (e.g., stride time CV: ρ = -0.57, p < 0.0001).
Discussion: We here identified trial-ready digital-motor candidate outcomes for the spastic ataxia SPG7 with proven multicenter applicability, ability to discriminate patients from controls, and correlation with measures of patient-relevant health aspects-even in mild disease stages. If validated longitudinally, these sensor outcomes might inform future natural history and treatment trials in SPG7 and other spastic ataxias.
Conflict of interest statement
L. Beichert, J. Seemann, C. Keßler, A. Traschütz, D. Müller, K. Dillmann-Jehn, I. Ricca, S. Satolli, A.N. Başak, G. Coarelli, D. Timmann and C. Gagnon report no disclosures relevant to the manuscript. B.P.C. van de Warrenburg receives research support from ZonMw, the Netherlands Organization for Scientific Research, Hersenstichting, Christina Foundation, and Radboud University Medical Center, has served on scientific advisory boards or provided consultancy services to Vico Therapeutics, Servier, and Biohaven Pharmaceuticals, and has received royalties from BSL-Springer Nature and speaker fees from MDC Malaysia. W. Ilg received consultancy honoraria from Ionis Pharmaceuticals. M. Synofzik has received consultancy honoraria from Ionis, UCB, Prevail, Orphazyme, Servier, Reata, GenOrph, AviadoBio, Biohaven, Zevra, and Lilly, all unrelated to the present manuscript. R. Schüle reports no disclosures relevant to the manuscript. Go to
Figures

References
-
- Casari G, Marconi R. Spastic paraplegia 7. In: Adam MP, Feldman J, Mirzaa GM, et al., eds. GeneReviews®. University of Washington, Seattle; 1993. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
