Integrating binding affinity and tonic signaling enables a rational CAR design for augmented T cell function
- PMID: 39622582
- PMCID: PMC11624832
- DOI: 10.1136/jitc-2024-010208
Integrating binding affinity and tonic signaling enables a rational CAR design for augmented T cell function
Abstract
Background: The success of chimeric antigen receptor (CAR) T cell therapy for hematological malignancies has not yet translated into long-term elimination of solid tumors indicating the need for adequately tuning CAR T cell functionality.
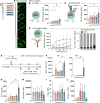
Methods: We leveraged a translational pipeline including biophysical characterization and structural prediction of the CAR binding moiety, evaluation of cellular avidity, synapse formation, T cell motility, and functional capacities under repetitive target challenge and in sustained tumor control.
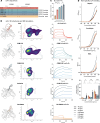
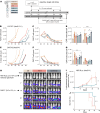
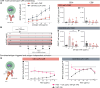
Results: As an example of clinical relevance, we derived a panel of anti-Her2 CARs covering a 4-log affinity range, all expected to target the same Her2 epitope. The same scFv mutations increased both antigen-specific affinity, cellular avidity, and antigen-independent "tonic" signaling; above a minimum threshold, raise in affinity translated into functional avidity in a non-linear fashion. In this case, replacement by amino acids of higher hydrophobicity within the scFv coincidentally augmented affinity, non-specific binding, spontaneous CAR clustering, and tonic signaling, all together relating to T cell functionality in an integrated fashion.
Conclusions: Data emphasize that tonic signaling is not always due to the positive charge but can be driven by hydrophobic interactions of the scFv. CAR binding affinity above the threshold and tonic signaling are required for sustained T cell functionality in antigen rechallenge and long-term tumor control.
Keywords: Chimeric antigen receptor - CAR; Immunotherapy; T cell.
© Author(s) (or their employer(s)) 2024. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: No, there are no competing interests.
Figures


References
-
- Mezősi-Csaplár M, Szöőr Á, Vereb G. CD28 and 41BB Costimulatory Domains Alone or in Combination Differentially Influence Cell Surface Dynamics and Organization of Chimeric Antigen Receptors and Early Activation of CAR T Cells. Cancers (Basel) 2023;15:3081. doi: 10.3390/cancers15123081. - DOI - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
