Neuropeptidergic Input from the Lateral Hypothalamus to the Suprachiasmatic Nucleus Alters the Circadian Period in Mice
- PMID: 39622648
- PMCID: PMC11756623
- DOI: 10.1523/JNEUROSCI.0351-24.2024
Neuropeptidergic Input from the Lateral Hypothalamus to the Suprachiasmatic Nucleus Alters the Circadian Period in Mice
Abstract
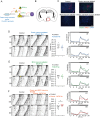
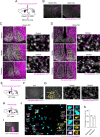
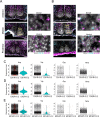
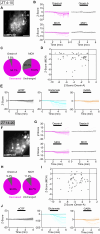
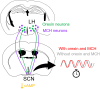
In mammals, the central circadian clock is located in the suprachiasmatic nucleus (SCN) of the hypothalamus, which transmits circadian information to other brain regions and regulates the timing of sleep and wakefulness. Neurons in the lateral hypothalamus (LH), particularly those producing melanin-concentrating hormone (MCH) and orexin, are key regulators of sleep and wakefulness. Although the SCN receives nonphotic input from other brain regions, the mechanisms of functional input from the LH to the SCN remain poorly understood. Here, we show that orexin and MCH peptides influence the circadian period within the SCN of both sexes. When these neurons are ablated, the circadian behavioral rhythms are lengthened under constant darkness. Using anterograde and retrograde tracing, we found that orexin and MCH neurons project to the SCN. Furthermore, the application of these peptides to cultured SCN slices shortened circadian rhythms and reduced intracellular cAMP levels. Additionally, pharmacological reduction of intracellular cAMP levels similarly shortened the circadian period in SCN slices. These findings suggest that orexin and MCH peptides from the LH contribute to the modulation of the circadian period in the SCN.
Keywords: cAMP; circadian rhythm; lateral hypothalamus; neuropeptides; suprachiasmatic nucleus.
Copyright © 2024 the authors.
Conflict of interest statement
The authors declare no competing financial interests.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
