Efficacy and Safety of Montelukast+Levocetirizine Combination Therapy Compared to Montelukast Monotherapy for Allergic Rhinitis in Children
- PMID: 39622689
- PMCID: PMC11621474
- DOI: 10.4168/aair.2024.16.6.652
Efficacy and Safety of Montelukast+Levocetirizine Combination Therapy Compared to Montelukast Monotherapy for Allergic Rhinitis in Children
Abstract
Purpose: The combination therapy of leukotriene receptor antagonists and antihistamines may alleviate allergic rhinitis (AR) symptoms better than monotherapy. This study aimed to investigate the safety and efficacy of Monterizine®, a fixed-dose combination of montelukast and levocetirizine, compared to montelukast monotherapy in pediatric patients with AR.
Methods: One hundred seventy-six children aged 6 to 14 years with perennial AR symptoms were recruited. One hundred forty-seven subjects were randomized into 1 of 2 groups: the mont+levo group (fixed-dose combination of montelukast [5 mg] + levocetirizine [5 mg]) or the mont group (montelukast single agent [5 mg]). Study subjects took the treatment every evening for 4 weeks and recorded their daytime nasal symptom score (DNSS) and nighttime nasal symptom score (NNSS) in a diary every day. Adverse events (AEs) were also recorded, and patients were surveyed as to their overall satisfaction with the therapeutic product they received.
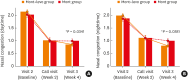
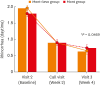
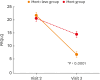
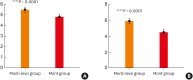
Results: When DNSS and NNSS were assessed individually, daytime nasal congestion symptom scores decreased more in the mont+levo group during the 4-week treatment period than in the mont group (P = 0.0341). The daytime rhinorrhea symptom scores also decreased more in the mont+levo group (P = 0.0469). The nighttime nasal congestion score (severity when awake) decreased more in the mont+levo group than in the mont group (P = 0.0381). Study subjects in the mont+levo group experienced a greater improvement in quality of life than subjects in the mont group (P < 0.0001).
Conclusions: The combination therapy of montelukast and levocetirizine was more effective in reducing both daytime nasal symptoms (nasal congestion and rhinorrhea) and nighttime nasal symptoms (severity of nasal congestion when awake). With fewer AEs and higher overall satisfaction, combination therapy is recommended for pediatric patients with perennial AR.
Keywords: Rhinitis, allergic; cetirizine; child; drug combinations; histamine antagonists; leukotriene antagonists; montelukast.
Copyright © 2024 The Korean Academy of Asthma, Allergy and Clinical Immunology · The Korean Academy of Pediatric Allergy and Respiratory Disease.
Conflict of interest statement
There are no financial or other issues that might lead to conflict of interest.
Figures

References
-
- Shamji MH, Durham SR. Mechanisms of allergen immunotherapy for inhaled allergens and predictive biomarkers. J Allergy Clin Immunol. 2017;140:1485–1498. - PubMed
-
- Pawankar R. Allergic rhinitis and asthma: the link, the new ARIA classification and global approaches to treatment. Curr Opin Allergy Clin Immunol. 2004;4:1–4. - PubMed
-
- Lack G. Pediatric allergic rhinitis and comorbid disorders. J Allergy Clin Immunol. 2001;108:S9–S15. - PubMed
-
- Navarro A, Valero A, Juliá B, Quirce S. Coexistence of asthma and allergic rhinitis in adult patients attending allergy clinics: ONEAIR study. J Investig Allergol Clin Immunol. 2008;18:233–238. - PubMed
-
- Liu W, Zeng Q, He C, Chen R, Tang Y, Yan S, et al. Compliance, efficacy, and safety of subcutaneous and sublingual immunotherapy in children with allergic rhinitis. Pediatr Allergy Immunol. 2021;32:86–91. - PubMed

