PET-CT outcomes from a randomised controlled trial of rosuvastatin as an adjunct to standard tuberculosis treatment
- PMID: 39622823
- PMCID: PMC11611914
- DOI: 10.1038/s41467-024-54419-3
PET-CT outcomes from a randomised controlled trial of rosuvastatin as an adjunct to standard tuberculosis treatment
Abstract
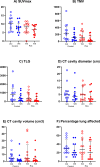
Adjunctive rosuvastatin for rifampicin-susceptible pulmonary tuberculosis (rs-PTB) shows no effect on microbiological or radiological outcomes in a phase IIb randomised, controlled trial (NCT04504851). We explore the impact of adjunctive rosuvastatin on 18F-fluorodeoxyglucose (FDG) positron emission tomography-computed tomography (PET-CT) imaging in a sub-study of 24 participants. Changes in standardised uptake value (SUVmax, SUVmean), Total Metabolic Volume, (TMV), Total Lesion Glycolysis (TLG), cavity diameter and volume, between week 0 and week 8 post-randomisation, are evaluated. Here we show no evidence of difference in the reduction in TLG [median 65.8% for the rosuvastatin group (Q1, Q3 38.6, 94.5) vs 64.3% for standard tuberculosis treatment group (Q1, Q3 -20.0, 81.7), P = 0.32], reduction in cavity volume on CT [median 3.2 cm3 (IQR 11.1, 0.5) for rosuvastatin, 2.2 cm3 (IQR 4.6, 0.7) for control (p = 0.72)], or any other PET-CT parameter measured. We show that the first 8-weeks of standard tuberculosis treatment results in a reduction in the volumetric indices (TLG and TMV), but had little change in SUVmax or SUVmean. Change in TLG and TMV holds promise as biomarkers of tuberculosis treatment response: future PET-CT studies should evaluate their role in predicting relapse-free cure, and the overall role of 18F-FDG-PET-CT as a tool for early-phase tuberculosis clinical trials.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: Dr. Jim O’Doherty is employed by Siemens Medical Solutions. However, Siemens Medical Solutions had no role in the conception, design, execution, data analysis, manuscript preparation, or the decision to submit this study for publication. The views expressed in this study are solely those of the authors and do not necessarily reflect those of Siemens Medical Solutions. All other authors declare no financial or non-financial competing interests.
Figures


References
-
- Parihar, S. P. et al. Statin therapy reduces the mycobacterium tuberculosis burden in human macrophages and in mice by enhancing autophagy and phagosome maturation. J. Infect. Dis.209, 754–763 (2014). - PubMed
-
- Lai, C. C. et al. Statin treatment is associated with a decreased risk of active tuberculosis: an analysis of a nationally representative cohort. Thorax71, 646–651 (2016). - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

