Development of a novel gut microphysiological system that facilitates assessment of drug absorption kinetics in gut
- PMID: 39622870
- PMCID: PMC11612460
- DOI: 10.1038/s41598-024-80946-6
Development of a novel gut microphysiological system that facilitates assessment of drug absorption kinetics in gut
Abstract
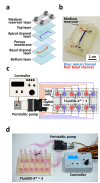
There is an urgent need for novel methods that can accurately predict intestinal absorption of orally administered drugs in humans. This study aimed to evaluate the potential of a novel gut microphysiological system (MPS), gut MPS/Fluid3D-X, to assess the intestinal absorption of drugs in humans. The gut MPS/Fluid3D-X model was constructed using a newly developed flow-controllable and dimethylpolysiloxane-free MPS device (Fluid3D-X®). Human induced pluripotent stem cells-derived small intestinal epithelial cells were employed in this model, which exhibited key characteristics of the human absorptive epithelial cells of the small intestine, including the expression of key gene transcripts responsible for drug transport and metabolism, and the presence of dome-like protrusions in the primary intestinal epithelium under air-liquid interface culture conditions. Functional studies of transporters in the constructed model demonstrated basal-to-apical directional transport of sulfasalazine and quinidine, substrates of the active efflux transporters breast cancer resistance protein and P-glycoprotein, respectively, which were diminished by inhibitors. Furthermore, a cytochrome P450 (CYP) 3A inhibitor increased the apical-to-basal transport of midazolam, a typical CYP3A4 substrate, and reduced metabolite formation. These results suggest that gut MPS/Fluid3D-X has the potential to assess the intestinal absorption of small-molecule drugs.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Disclosures: Tomoki Imaoka, Misa Hoshi and Kengo Watanabe are employees of Daiichi Sankyo Co., Ltd.
Figures



References
-
- Zhang, H. et al. Regional Proteomic quantification of clinically relevant Non-cytochrome P450 enzymes along the human small intestine. Drug Metab. Dispos.48, 528–536. 10.1124/dmd.120.090738 (2020). - PubMed
-
- Sietsema, W. K. The absolute oral bioavailability of selected drugs. Int. J. Clin. Pharmacol. Ther. Toxicol.27, 179–211 (1989). - PubMed
-
- Takahashi, M., Washio, T., Suzuki, N., Igeta, K. & Yamashita, S. The species differences of intestinal drug absorption and first-pass metabolism between cynomolgus monkeys and humans. J. Pharm. Sci.98, 4343–4353. 10.1002/jps.21708 (2009). - PubMed
MeSH terms
Substances
Grants and funding
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
- JP17be0304204, JP17be0304101, P22be1004101 and JP22be1004301/The Japanese Agency for Medical Research and Development
LinkOut - more resources
Full Text Sources

